Association Between Human Immunodeficiency Virus Viremia and Compromised Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2 Beta Variant
- PMID: 35975942
- PMCID: PMC9452105
- DOI: 10.1093/infdis/jiac343
Association Between Human Immunodeficiency Virus Viremia and Compromised Neutralization of Severe Acute Respiratory Syndrome Coronavirus 2 Beta Variant
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may be associated with worse clinical outcomes in people with human immunodeficiency virus (HIV) (PWH). We report anti-SARS-CoV-2 antibody responses in patients hospitalized with coronavirus disease 2019 in Durban, South Africa, during the second SARS-CoV-2 infection wave dominated by the Beta (B.1.351) variant.
Methods: Thirty-four participants with confirmed SARS-CoV-2 infection were followed up with weekly blood sampling to examine antibody levels and neutralization potency against SARS-CoV-2 variants. Participants included 18 PWH, of whom 11 were HIV viremic.
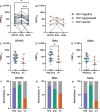
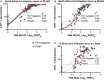
Results: SARS-CoV-2-specific antibody concentrations were generally lower in viremic PWH than in virologically suppressed PWH and HIV-negative participants, and neutralization of the Beta variant was 4.9-fold lower in viremic PWH. Most HIV-negative participants and antiretroviral therapy-suppressed PWH also neutralized the Delta (B.1.617.2) variant, whereas the majority of viremic PWH did not. CD4 cell counts <500/μL were associated with lower frequencies of immunoglobulin G and A seroconversion. In addition, there was a high correlation between a surrogate virus neutralization test and live virus neutralization against ancestral SARS-CoV-2 virus in both PWH and HIV-negative individuals, but correlation decreased for the Beta variant neutralization in PWH.
Conclusions: HIV viremia was associated with reduced Beta variant neutralization. This highlights the importance of HIV suppression in maintaining an effective SARS-CoV-2 neutralization response.
Keywords: Beta variant; COVID-19; HIV; SARS-CoV-2; antibodies; antiretroviral therapy; neutralization.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. W. T. and L. F. W. report the following issued patent: US patent 11054429 B1 (SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein binding). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Tegally H, Wilkinson E, Giovanetti M, et al. . Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021; 592:438–43. - PubMed
-
- Wibmer CK, Ayres F, Hermanus T, et al. . SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 2021; 27:622–5. - PubMed
-
- Madhi SA, Koen AL, Izu A, et al. . Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021; 8:e568–80. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

