Artifact suppression in readout-segmented consistent K-t space EPSI (RS-COKE) for fast 1 H spectroscopic imaging at 7 T
- PMID: 35975965
- PMCID: PMC9804880
- DOI: 10.1002/mrm.29373
Artifact suppression in readout-segmented consistent K-t space EPSI (RS-COKE) for fast 1 H spectroscopic imaging at 7 T
Abstract
Purpose: Fast proton (1 H) MRSI is an important diagnostic tool for clinical investigations, providing metabolic and spatial information. MRSI at 7 T benefits from increased SNR and improved separation of peaks but requires larger spectral widths. RS-COKE (Readout-Segmented Consistent K-t space Epsi) is an echo planar spectroscopic imaging (Epsi) variant capable to support the spectral width required for human brain metabolites spectra at 7 T. However, mismatches between readout segments lead to artifacts, particularly when subcutaneous lipid signals are not suppressed. In this study, these mismatches and their effects are analyzed and reduced.
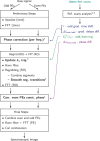
Methods: The following corrections to the data were performed: i) frequency-dependent phase corrections; ii) k-space trajectory corrections, derived from short reference scans; and iii) smoothing of data at segment transitions to mitigate the effect of residual mismatches. The improvement was evaluated by performing single-slice RS-COKE on a head-shaped phantom with a "lipid" layer and healthy subjects, using varying resolutions and durations ranging from 4.1 × 4.7 × 15 mm3 in 5:46 min to 3.1 × 3.3 × 15 mm3 in 13:07 min.
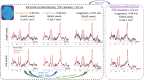
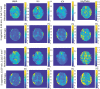
Results: Artifacts arising from the readout-segmented acquisition were substantially reduced, thus providing high-quality spectroscopic imaging in phantom and human scans. LCModel fitting of the human data resulted in a relative Cramer-Rao lower bounds within 6% for NAA, Cr, and Cho images in the majority of the voxels.
Conclusion: Using the new reference scans and reconstruction steps, RS-COKE was able to deliver fast 1 H MRSI at 7 T, overcoming the spectral width limitation of standard EPSI at this field strength.
Keywords: MRSI; spectroscopic imaging; ultrahigh field.
© 2022 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
A.S. is employed by Siemens Healthcare Ltd, Israel; all other authors declare no competing financial interests. 10‐Jun‐2022.
Figures







References
-
- Gillies RJ, Morse DL. In vivo magnetic resonance spectroscopy in cancer. Annu Rev Biomed Eng. 2005;7:287‐326. - PubMed
-
- Keshavan MS, Stanley JA, Pettegrew JW. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings—part II. Biol Psychiatry. 2000;48:369‐380. - PubMed
-
- Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47:305‐313. - PubMed

