Inflammation and Metabolism of Influenza-Stimulated Peripheral Blood Mononuclear Cells From Adults With Obesity Following Bariatric Surgery
- PMID: 35975968
- PMCID: PMC10205606
- DOI: 10.1093/infdis/jiac345
Inflammation and Metabolism of Influenza-Stimulated Peripheral Blood Mononuclear Cells From Adults With Obesity Following Bariatric Surgery
Abstract
Background: Obesity dysregulates immunity to influenza infection. Therefore, there is a critical need to investigate how obesity impairs immunity and to establish therapeutic approaches that mitigate the impact of increased adiposity. One mechanism by which obesity may alter immune responses is through changes in cellular metabolism.
Methods: We studied inflammation and cellular metabolism of peripheral blood mononuclear cells (PBMCs) isolated from individuals with obesity relative to lean controls. We also investigated if impairments to PBMC metabolism were reversible upon short-term weight loss following bariatric surgery.
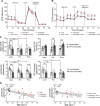
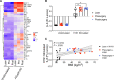
Results: Obesity was associated with systemic inflammation and poor inflammation resolution. Unstimulated PBMCs from participants with obesity had lower oxidative metabolism and adenosine triphosphate (ATP) production compared to PBMCs from lean controls. PBMC secretome analyses showed that ex vivo stimulation with A/Cal/7/2009 H1N1 influenza led to a notable increase in IL-6 with obesity. Short-term weight loss via bariatric surgery improved biomarkers of systemic metabolism but did not improve markers of inflammation resolution, PBMC metabolism, or the PBMC secretome.
Conclusions: These results show that obesity drives a signature of impaired PBMC metabolism, which may be due to persistent inflammation. PBMC metabolism was not reversed after short-term weight loss despite improvements in measures of systemic metabolism.
Keywords: bariatric surgery; immunometabolism; inflammation; influenza virus.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


Similar articles
-
Reduced Th1 response is associated with lower glycolytic activity in activated peripheral blood mononuclear cells after metabolic and bariatric surgery.J Endocrinol Invest. 2021 Dec;44(12):2819-2830. doi: 10.1007/s40618-021-01587-4. Epub 2021 May 15. J Endocrinol Invest. 2021. PMID: 33991317
-
Response to immune checkpoint blockade improved in pre-clinical model of breast cancer after bariatric surgery.Elife. 2022 Jul 1;11:e79143. doi: 10.7554/eLife.79143. Elife. 2022. PMID: 35775614 Free PMC article.
-
Weight loss normalizes enhanced expression of the oncogene survivin in visceral adipose tissue and blood leukocytes from individuals with obesity.Int J Obes (Lond). 2021 Jan;45(1):206-216. doi: 10.1038/s41366-020-0630-7. Epub 2020 Jun 16. Int J Obes (Lond). 2021. PMID: 32546857 Free PMC article.
-
Potential mechanisms by which bariatric surgery improves systemic metabolism.Transl Res. 2013 Feb;161(2):63-72. doi: 10.1016/j.trsl.2012.09.004. Epub 2012 Oct 16. Transl Res. 2013. PMID: 23079469 Review.
-
Interplay between the Adaptive Immune System and Insulin Resistance in Weight Loss Induced by Bariatric Surgery.Oxid Med Cell Longev. 2019 Dec 6;2019:3940739. doi: 10.1155/2019/3940739. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31885787 Free PMC article. Review.
Cited by
-
Diet switch pre-vaccination improves immune response and metabolic status in formerly obese mice.Nat Microbiol. 2024 Jun;9(6):1593-1606. doi: 10.1038/s41564-024-01677-y. Epub 2024 Apr 18. Nat Microbiol. 2024. PMID: 38637722
-
Emerging mechanisms of obesity-associated immune dysfunction.Nat Rev Endocrinol. 2024 Mar;20(3):136-148. doi: 10.1038/s41574-023-00932-2. Epub 2023 Dec 21. Nat Rev Endocrinol. 2024. PMID: 38129700 Review.
-
Impact of bariatric surgery on gut microbiota in obese patients: A systematic review.Indian J Gastroenterol. 2025 Aug;44(4):457-477. doi: 10.1007/s12664-025-01763-x. Epub 2025 Apr 12. Indian J Gastroenterol. 2025. PMID: 40220249
References
-
- Louie JK, Acosta M, Samuel MC, et al. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011; 52:301–12. - PubMed
-
- National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID-19): people who are at higher risk for severe illness, 2022. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

