Aminomethanesulfonic acid illuminates the boundary between full and partial agonists of the pentameric glycine receptor
- PMID: 35975975
- PMCID: PMC9462852
- DOI: 10.7554/eLife.79148
Aminomethanesulfonic acid illuminates the boundary between full and partial agonists of the pentameric glycine receptor
Abstract
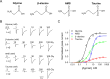
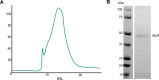
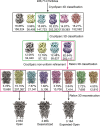
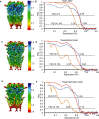
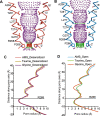
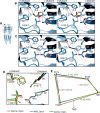
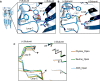
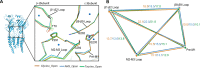
To clarify the determinants of agonist efficacy in pentameric ligand-gated ion channels, we examined a new compound, aminomethanesulfonic acid (AMS), a molecule intermediate in structure between glycine and taurine. Despite wide availability, to date there are no reports of AMS action on glycine receptors, perhaps because AMS is unstable at physiological pH. Here, we show that at pH 5, AMS is an efficacious agonist, eliciting in zebrafish α1 glycine receptors a maximum single-channel open probability of 0.85, much greater than that of β-alanine (0.54) or taurine (0.12), and second only to that of glycine itself (0.96). Thermodynamic cycle analysis of the efficacy of these closely related agonists shows supra-additive interaction between changes in the length of the agonist molecule and the size of the anionic moiety. Single particle cryo-electron microscopy structures of AMS-bound glycine receptors show that the AMS-bound agonist pocket is as compact as with glycine, and three-dimensional classification demonstrates that the channel populates the open and the desensitized states, like glycine, but not the closed intermediate state associated with the weaker partial agonists, β-alanine and taurine. Because AMS is on the cusp between full and partial agonists, it provides a new tool to help us understand agonist action in the pentameric superfamily of ligand-gated ion channels.
Keywords: agonists; cryoEM; glycine receptors; molecular biophysics; single channel recording; structural biology; zebrafish.
© 2022, Ivica, Zhu et al.
Conflict of interest statement
JI, HZ, RL, EG, LS No competing interests declared
Figures



Similar articles
-
The intracellular domain of homomeric glycine receptors modulates agonist efficacy.J Biol Chem. 2021 Jan-Jun;296:100387. doi: 10.1074/jbc.RA119.012358. Epub 2021 Feb 20. J Biol Chem. 2021. PMID: 33617876 Free PMC article.
-
Acidic pH reduces agonist efficacy and responses to synaptic-like glycine applications in zebrafish α1 and rat α1β recombinant glycine receptors.J Physiol. 2022 Jan;600(2):333-347. doi: 10.1113/JP282171. Epub 2021 Dec 8. J Physiol. 2022. PMID: 34802146 Free PMC article.
-
Mechanism of gating and partial agonist action in the glycine receptor.Cell. 2021 Feb 18;184(4):957-968.e21. doi: 10.1016/j.cell.2021.01.026. Epub 2021 Feb 9. Cell. 2021. PMID: 33567265 Free PMC article.
-
What single-channel analysis tells us of the activation mechanism of ligand-gated channels: the case of the glycine receptor.J Physiol. 2010 Jan 1;588(Pt 1):45-58. doi: 10.1113/jphysiol.2009.178525. Epub 2009 Sep 21. J Physiol. 2010. PMID: 19770192 Free PMC article. Review.
-
Building new function into glycine receptors: a structural model for the activation of the glycine-gated chloride channel.Clin Exp Pharmacol Physiol. 1999 Nov;26(11):932-4. doi: 10.1046/j.1440-1681.1999.03150.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10561818 Review.
Cited by
-
Mechanistic basis of ligand efficacy in the calcium-activated chloride channel TMEM16A.EMBO J. 2023 Dec 11;42(24):e115030. doi: 10.15252/embj.2023115030. Epub 2023 Nov 20. EMBO J. 2023. PMID: 37984335 Free PMC article.
-
Manure odor profiling for flock-level monitoring on commercial layer pullet farms: Vaccination events as a model stressor.Poult Sci. 2025 Feb;104(2):104681. doi: 10.1016/j.psj.2024.104681. Epub 2024 Dec 16. Poult Sci. 2025. PMID: 39721281 Free PMC article.
-
Asymmetric gating of a human hetero-pentameric glycine receptor.Nat Commun. 2023 Oct 11;14(1):6377. doi: 10.1038/s41467-023-42051-6. Nat Commun. 2023. PMID: 37821459 Free PMC article.
References
-
- Benoit RL, Boulet D, Fréchette M. Solvent effect on the solution, ionization, and structure of aminosulfonic acids. Canadian Journal of Chemistry. 1988;66:3038–3043. doi: 10.1139/v88-470. - DOI
-
- Burzomato V, Beato M, Groot-Kormelink PJ, Colquhoun D, Sivilotti LG. Single-channel behavior of heteromeric alpha1beta glycine receptors: an attempt to detect a conformational change before the channel opens. The Journal of Neuroscience. 2004;24:10924–10940. doi: 10.1523/JNEUROSCI.3424-04.2004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

