Biphasic regulation of osteoblast development via the ERK MAPK-mTOR pathway
- PMID: 35975983
- PMCID: PMC9417416
- DOI: 10.7554/eLife.78069
Biphasic regulation of osteoblast development via the ERK MAPK-mTOR pathway
Abstract
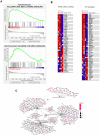
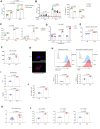
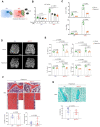
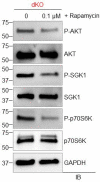
Emerging evidence supports that osteogenic differentiation of skeletal progenitors is a key determinant of overall bone formation and bone mass. Despite extensive studies showing the function of mitogen-activated protein kinases (MAPKs) in osteoblast differentiation, none of these studies show in vivo evidence of a role for MAPKs in osteoblast maturation subsequent to lineage commitment. Here, we describe how the extracellular signal-regulated kinase (ERK) pathway in osteoblasts controls bone formation by suppressing the mechanistic target of rapamycin (mTOR) pathway. We also show that, while ERK inhibition blocks the differentiation of osteogenic precursors when initiated at an early stage, ERK inhibition surprisingly promotes the later stages of osteoblast differentiation. Accordingly, inhibition of the ERK pathway using a small compound inhibitor or conditional deletion of the MAP2Ks Map2k1 (MEK1) and Map2k2 (MEK2), in mature osteoblasts and osteocytes, markedly increased bone formation due to augmented osteoblast differentiation. Mice with inducible deletion of the ERK pathway in mature osteoblasts also displayed similar phenotypes, demonstrating that this phenotype reflects continuous postnatal inhibition of late-stage osteoblast maturation. Mechanistically, ERK inhibition increases mitochondrial function and SGK1 phosphorylation via mTOR2 activation, which leads to osteoblast differentiation and production of angiogenic and osteogenic factors to promote bone formation. This phenotype was partially reversed by inhibiting mTOR. Our study uncovers a surprising dichotomy of ERK pathway functions in osteoblasts, whereby ERK activation promotes the early differentiation of osteoblast precursors, but inhibits the subsequent differentiation of committed osteoblasts via mTOR-mediated regulation of mitochondrial function and SGK1.
Keywords: ERK; MEK; mTOR; medicine; mouse; osteoblast.
© 2022, Kim et al.
Conflict of interest statement
JK, YY, JH, SC, HC, Mv, RX, MG No competing interests declared, JS is a scientific co-founder of the AAVAA Therapeutics and holds equity in this company. These pose no conflicts for this study
Figures
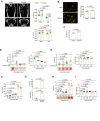


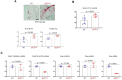

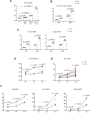
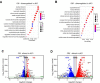




Similar articles
-
The ERK MAPK Pathway Is Essential for Skeletal Development and Homeostasis.Int J Mol Sci. 2019 Apr 12;20(8):1803. doi: 10.3390/ijms20081803. Int J Mol Sci. 2019. PMID: 31013682 Free PMC article.
-
TGF-β inhibits osteogenesis by upregulating the expression of ubiquitin ligase SMURF1 via MAPK-ERK signaling.J Cell Physiol. 2018 Jan;233(1):596-606. doi: 10.1002/jcp.25920. Epub 2017 Jun 5. J Cell Physiol. 2018. PMID: 28322449
-
Osteoblast-targeted suppression of PPARγ increases osteogenesis through activation of mTOR signaling.Stem Cells. 2013 Oct;31(10):2183-92. doi: 10.1002/stem.1455. Stem Cells. 2013. PMID: 23766271 Free PMC article.
-
EGFR controls bone development by negatively regulating mTOR-signaling during osteoblast differentiation.Cell Death Differ. 2018 Jun;25(6):1094-1106. doi: 10.1038/s41418-017-0054-7. Epub 2018 Feb 14. Cell Death Differ. 2018. PMID: 29445126 Free PMC article.
-
Mitogen-activated protein kinase pathways in osteoblasts.Annu Rev Cell Dev Biol. 2013;29:63-79. doi: 10.1146/annurev-cellbio-101512-122347. Epub 2013 May 31. Annu Rev Cell Dev Biol. 2013. PMID: 23725048 Review.
Cited by
-
Bone repair and key signalling pathways for cell-based bone regenerative therapy: A review.J Taibah Univ Med Sci. 2023 May 24;18(6):1350-1363. doi: 10.1016/j.jtumed.2023.05.015. eCollection 2023 Dec. J Taibah Univ Med Sci. 2023. PMID: 37305024 Free PMC article. Review.
-
Skeletal health in DYRK1A syndrome.Front Neurosci. 2024 Sep 6;18:1462893. doi: 10.3389/fnins.2024.1462893. eCollection 2024. Front Neurosci. 2024. PMID: 39308945 Free PMC article. Review.
-
MK-4 Ameliorates Diabetic Osteoporosis in Angiogenesis-Dependent Bone Formation by Promoting Mitophagy in Endothelial Cells.Drug Des Devel Ther. 2025 Mar 25;19:2173-2188. doi: 10.2147/DDDT.S503930. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 40160965 Free PMC article.
-
Apoptotic Vesicles Derived from Dental Pulp Stem Cells Promote Bone Formation through the ERK1/2 Signaling Pathway.Biomedicines. 2024 Mar 25;12(4):730. doi: 10.3390/biomedicines12040730. Biomedicines. 2024. PMID: 38672086 Free PMC article.
-
Unraveling the Mechanism of Impaired Osteogenic Differentiation in Osteoporosis: Insights from ADRB2 Gene Polymorphism.Cells. 2024 Dec 20;13(24):2110. doi: 10.3390/cells13242110. Cells. 2024. PMID: 39768200 Free PMC article.
References
-
- Bado IL, Zhang W, Hu J, Xu Z, Wang H, Sarkar P, Li L, Wan YW, Liu J, Wu W, Lo HC, Kim IS, Singh S, Janghorban M, Muscarella AM, Goldstein A, Singh P, Jeong HH, Liu C, Schiff R, Huang S, Ellis MJ, Gaber MW, Gugala Z, Liu Z, Zhang XHF. The bone microenvironment increases phenotypic plasticity of ER+ breast cancer cells. Developmental Cell. 2021;56:1100–1117. doi: 10.1016/j.devcel.2021.03.008. - DOI - PMC - PubMed
-
- Bateman JF, Sampurno L, Maurizi A, Lamandé SR, Sims NA, Cheng TL, Schindeler A, Little DG. Effect of rapamycin on bone mass and strength in the α2(I)-G610C mouse model of osteogenesis imperfecta. Journal of Cellular and Molecular Medicine. 2019;23:1735–1745. doi: 10.1111/jcmm.14072. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

