Do Liquid Nitrogen-treated Tumor-bearing Nerve Grafts Have the Capacity to Regenerate, and Do They Pose a Risk of Local Recurrence? A Study in Rats
- PMID: 35976198
- PMCID: PMC10540061
- DOI: 10.1097/CORR.0000000000002336
Do Liquid Nitrogen-treated Tumor-bearing Nerve Grafts Have the Capacity to Regenerate, and Do They Pose a Risk of Local Recurrence? A Study in Rats
Abstract
Background: Under most circumstances, the resection of soft tissue sarcomas of the extremities can be limb-sparing, function-preserving oncologic resections with adequate margins. However, en bloc resection may require resection of the major peripheral nerves, causing poor function in the extremities. Although liquid nitrogen treatment has been used to sterilize malignant bone tumors, its use in the preparation of nerve grafts has, to our knowledge, not been reported. Hence, this study aimed to investigate the tumor recurrence and function after peripheral nerve reconstruction using liquid nitrogen-treated tumor-bearing nerves in a rat model.
Questions/purposes: (1) Do liquid nitrogen-treated frozen autografts have regeneration capabilities? (2) Do liquid nitrogen-treated tumor-bearing nerves cause any local recurrences in vivo in a rat model?
Methods: Experiment 1: Twelve-week-old female Wistar rats, each weighing 250 g to 300 g, were used. A 10-mm-long section of the right sciatic nerve was excised; the prepared nerve grafts were bridge-grafted through end-to-end suturing. The rats were grouped as follows: an autograft group, which underwent placement of a resected sciatic nerve after it was sutured in the reverse orientation, and a frozen autograft group, which underwent bridging of the nerve gap using a frozen autograft. The autograft was frozen in liquid nitrogen, thawed at room temperature, and then thawed in distilled water before application. The third group was a resection group in which the nerve gap was not reconstructed. Twenty-four rats were included in each group, and six rats per group were evaluated at 4, 12, 24, and 48 weeks postoperatively. To assess nerve regeneration after reconstruction using the frozen nerve graft in the nontumor rat model, we evaluated the sciatic functional index, tibialis anterior muscle wet weight ratio, electrophysiologic parameters (amplitude and latency), muscle fiber size (determined with Masson trichrome staining), lower limb muscle volume, and immunohistochemical findings (though neurofilament staining and S100 protein produced solely and uniformly by Schwann cells associated with axons). Lower limb muscle volume was calculated via CT before surgery (0 weeks) and at 4, 8, 12, 16, 20, 24, 32, 40, and 48 weeks after surgery. Experiment 2: Ten-week-old female nude rats (F344/NJcl-rnu/rnu rats), each weighing 100 g to 150 g, were injected with HT1080 (human fibrosarcoma) cells near the bilateral sciatic nerves. Two weeks after injection, the tumor grew to a 10-mm-diameter mass involving the sciatic nerves. Subsequently, the tumor was resected with the sciatic nerves, and tumor-bearing sciatic nerves were obtained. After liquid nitrogen treatment, the frozen tumor-bearing nerve graft was trimmed to a 5-mm-long tissue and implanted into another F344/NJcl-rnu/rnu rat, in which a 5-mm-long section of the sciatic nerve was resected to create a nerve gap. Experiment 2 was performed with 12 rats; six rats were evaluated at 24 and 48 weeks postoperatively. To assess nerve regeneration and tumor recurrence after nerve reconstruction using frozen tumor-bearing nerve grafts obtained from the nude rat with human fibrosarcoma involving the sciatic nerve, the sciatic nerve's function and histologic findings were evaluated in the same way as in Experiment 1.
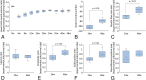
Results: Experiment 1: The lower limb muscle volume decreased once at 4 weeks in the autograft and frozen autograft groups and gradually increased thereafter. The tibialis anterior muscle wet weight ratio, sciatic functional index, muscle fiber size, and electrophysiologic evaluation showed higher nerve regeneration potential in the autograft and frozen autograft groups than in the resection group. The median S100-positive areas (interquartile range [IQR]) in the autograft group were larger than those in the frozen autograft group at 12 weeks (0.83 [IQR 0.78 to 0.88] versus 0.57 [IQR 0.53 to 0.61], difference of medians 0.26; p = 0.04) and at 48 weeks (0.86 [IQR 0.83 to 0.99] versus 0.74 [IQR 0.69 to 0.81], difference of median 0.12; p = 0.03). Experiment 2: Lower limb muscle volume decreased at 4 weeks and gradually increased thereafter. The median muscle fiber size increased from 0.89 (IQR 0.75 to 0.90) at 24 weeks to 1.20 (IQR 1.08 to 1.34) at 48 weeks (difference of median 0.31; p< 0.01). The median amplitude increased from 0.60 (IQR 0.56 to 0.67) at 24 weeks to 0.81 (IQR 0.76 to 0.90) at 48 weeks (difference of median 0.21; p < 0.01). Despite tumor involvement and freezing treatment, tumor-bearing frozen grafts demonstrated nerve regeneration activity, with no local recurrence observed at 48 weeks postoperatively in nude rats.
Conclusion: Tumor-bearing frozen nerve grafts demonstrated nerve regeneration activity, and there was no tumor recurrence in rats in vivo.
Clinical relevance: A frozen nerve autograft has a similar regenerative potential to that of a nerve autograft. Although the findings in a rat model do not guarantee efficacy in humans, if they are substantiated by large-animal models, clinical trials will be needed to evaluate the efficacy of tumor-bearing frozen nerve grafts in humans.
Copyright © 2022 by the Association of Bone and Joint Surgeons.
Conflict of interest statement
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members. All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Figures





Comment in
-
CORR Insights®: Do Liquid Nitrogen-treated Tumor-bearing Nerve Grafts Have the Capacity to Regenerate, and Do They Pose a Risk of Local Recurrence? A Study in Rats.Clin Orthop Relat Res. 2022 Dec 1;480(12):2456-2458. doi: 10.1097/CORR.0000000000002400. Epub 2022 Oct 10. Clin Orthop Relat Res. 2022. PMID: 36214780 Free PMC article. No abstract available.
Similar articles
-
No Difference in Outcomes Detected Between Decellular Nerve Allograft and Cable Autograft in Rat Sciatic Nerve Defects.J Bone Joint Surg Am. 2019 May 15;101(10):e42. doi: 10.2106/JBJS.18.00417. J Bone Joint Surg Am. 2019. PMID: 31094986
-
What Are the Challenges and Complications of Sterilizing Autografts with Liquid Nitrogen for Malignant Bone Tumors? A Preliminary Report.Clin Orthop Relat Res. 2020 Nov;478(11):2505-2519. doi: 10.1097/CORR.0000000000001347. Clin Orthop Relat Res. 2020. PMID: 32510187 Free PMC article.
-
Comparison of the Effects of Extracorporeal Irradiation and Liquid Nitrogen on Nerve Recovery in a Rat Model.J Invest Surg. 2021 Jul;34(7):773-783. doi: 10.1080/08941939.2019.1691686. Epub 2020 Feb 4. J Invest Surg. 2021. PMID: 32013622
-
Intercalary frozen autografts for reconstruction of bone defects following meta-/diaphyseal tumor resection at the extremities.BMC Musculoskelet Disord. 2022 Sep 30;23(1):890. doi: 10.1186/s12891-022-05840-6. BMC Musculoskelet Disord. 2022. PMID: 36180843 Free PMC article. Review.
-
The effectiveness of acellular nerve allografts compared to autografts in animal models: A systematic review and meta-analysis.PLoS One. 2024 Jan 31;19(1):e0279324. doi: 10.1371/journal.pone.0279324. eCollection 2024. PLoS One. 2024. PMID: 38295088 Free PMC article.
Cited by
-
CORR Insights®: Do Liquid Nitrogen-treated Tumor-bearing Nerve Grafts Have the Capacity to Regenerate, and Do They Pose a Risk of Local Recurrence? A Study in Rats.Clin Orthop Relat Res. 2022 Dec 1;480(12):2456-2458. doi: 10.1097/CORR.0000000000002400. Epub 2022 Oct 10. Clin Orthop Relat Res. 2022. PMID: 36214780 Free PMC article. No abstract available.
References
-
- Cancer Society American. Cancer facts and figures 2022. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts.... Accessed April 17, 2022.
-
- Ansselin AD, Davey DF. The regeneration of axons through normal and reversed peripheral nerve grafts. Restor Neurol Neurosci. 1993;5:225-240. - PubMed
-
- Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129-138. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

