Deep learning for standardized, MRI-based quantification of subcutaneous and subfascial tissue volume for patients with lipedema and lymphedema
- PMID: 35976393
- PMCID: PMC9889496
- DOI: 10.1007/s00330-022-09047-0
Deep learning for standardized, MRI-based quantification of subcutaneous and subfascial tissue volume for patients with lipedema and lymphedema
Abstract
Objectives: To contribute to a more in-depth assessment of shape, volume, and asymmetry of the lower extremities in patients with lipedema or lymphedema utilizing volume information from MR imaging.
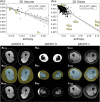
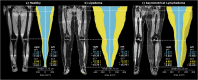
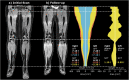
Methods: A deep learning (DL) pipeline was developed including (i) localization of anatomical landmarks (femoral heads, symphysis, knees, ankles) and (ii) quality-assured tissue segmentation to enable standardized quantification of subcutaneous (SCT) and subfascial tissue (SFT) volumes. The retrospectively derived dataset for method development consisted of 45 patients (42 female, 44.2 ± 14.8 years) who underwent clinical 3D DIXON MR-lymphangiography examinations of the lower extremities. Five-fold cross-validated training was performed on 16,573 axial slices from 40 patients and testing on 2187 axial slices from 5 patients. For landmark detection, two EfficientNet-B1 convolutional neural networks (CNNs) were applied in an ensemble. One determines the relative foot-head position of each axial slice with respect to the landmarks by regression, the other identifies all landmarks in coronal reconstructed slices using keypoint detection. After landmark detection, segmentation of SCT and SFT was performed on axial slices employing a U-Net architecture with EfficientNet-B1 as encoder. Finally, the determined landmarks were used for standardized analysis and visualization of tissue volume, distribution, and symmetry, independent of leg length, slice thickness, and patient position.
Results: Excellent test results were observed for landmark detection (z-deviation = 4.5 ± 3.1 mm) and segmentation (Dice score: SCT = 0.989 ± 0.004, SFT = 0.994 ± 0.002).
Conclusions: The proposed DL pipeline allows for standardized analysis of tissue volume and distribution and may assist in diagnosis of lipedema and lymphedema or monitoring of conservative and surgical treatments.
Key points: • Efficient use of volume information that MRI inherently provides can be extracted automatically by deep learning and enables in-depth assessment of tissue volumes in lipedema and lymphedema. • The deep learning pipeline consisting of body part regression, keypoint detection, and quality-assured tissue segmentation provides detailed information about the volume, distribution, and asymmetry of lower extremity tissues, independent of leg length, slice thickness, and patient position.
Keywords: Deep learning; Leg; Lymphography; Magnetic resonance imaging; Subcutaneous tissue.
© 2022. The Author(s).
Conflict of interest statement
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Figures

References
-
- Reich-Schupke S, Schmeller W, Brauer WJ, et al. S1 guidelines: lipedema. J Dtsch Dermatol Ges. 2017;15(7):758–767. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

