Pharmacokinetic/Pharmacodynamic Evaluation of the Dipeptidyl Peptidase 1 Inhibitor Brensocatib for Non-cystic Fibrosis Bronchiectasis
- PMID: 35976570
- PMCID: PMC9553789
- DOI: 10.1007/s40262-022-01147-w
Pharmacokinetic/Pharmacodynamic Evaluation of the Dipeptidyl Peptidase 1 Inhibitor Brensocatib for Non-cystic Fibrosis Bronchiectasis
Abstract
Background and objective: Brensocatib is an investigational, first-in-class, selective, and reversible dipeptidyl peptidase 1 inhibitor that blocks activation of neutrophil serine proteases (NSPs). The NSPs neutrophil elastase, cathepsin G, and proteinase 3 are believed to be central to the pathogenesis of several chronic inflammatory diseases, including bronchiectasis. In a phase II study, oral brensocatib 10 mg and 25 mg reduced sputum neutrophil elastase activity and prolonged the time to pulmonary exacerbation in patients with non-cystic fibrosis bronchiectasis (NCFBE). A population pharmacokinetic (PPK) model was developed to characterize brensocatib exposure, determine potential relationships between brensocatib exposure and efficacy and safety measures, and inform dose selection in clinical studies.
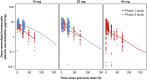
Methods: Pharmacokinetic (PK) data pooled from a phase I study of once-daily brensocatib (10, 25, and 40 mg) in healthy adults and a phase II study of once-daily brensocatib (10 mg and 25 mg) in adults with NCFBE were used to develop a PPK model and to evaluate potential covariate effects on brensocatib pharmacokinetics. PK-efficacy relationships for sputum neutrophil elastase below the level of quantification (BLQ) and reduction in pulmonary exacerbation and PK-safety relationships for adverse events of special interest (AESIs; periodontal disease, hyperkeratosis, and infections other than pulmonary infections) were evaluated based on model-predicted brensocatib exposure. A total of 1284 steady-state brensocatib concentrations from 225 individuals were included in the PPK data set; 241 patients with NCFBE from the phase II study were included in the pharmacodynamic (PD) population for the PK/PD analyses.
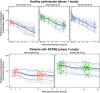
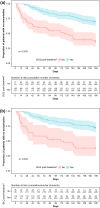
Results: The PPK model that best described the observed data consisted of two distributional compartments and linear clearance. Two significant covariates were found: age on volume of distribution and renal function on apparent oral clearance. PK-efficacy analysis revealed a threshold brensocatib exposure (area under the concentration-time curve) effect for attaining sputum neutrophil elastase BLQ and a strong relationship between sputum neutrophil elastase BLQ and reduction in pulmonary exacerbations. A PK-safety evaluation showed no noticeable trends between brensocatib exposure and the incidence of AESIs. Based on the predicted likelihood of clinical outcomes for sputum neutrophil elastase BLQ and pulmonary exacerbations, brensocatib doses of 10 mg and 25 mg once daily were selected for a phase III clinical trial in patients with NCFBE (ClinicalTrials.gov identifier: NCT04594369).
Conclusions: PPK results revealed that age and renal function have a moderate effect on brensocatib exposure. However, this finding does not warrant dose adjustments based on age or in those with mild or moderate renal impairment. The PK/PD evaluation demonstrated the clinically meaningful relationship between suppression of neutrophil elastase activity and reduction in exacerbations in brensocatib-treated patients with NCFBE, supporting further development of brensocatib for bronchiectasis.
© 2022. The Author(s).
Conflict of interest statement
Institute for Clinical Pharmacodynamics (ICPD), Inc. received funding from Insmed Incorporated to conduct the analyses and provide general consulting to Insmed Incorporated. Helen Usansky, Carlos Fernandez, Ariel Teper, Jun Zou, and Kevin C. Mange are employees of and shareholders in Insmed Incorporated. Christopher M. Rubino is an employee of ICPD, Inc. James D. Chalmers has received grants and personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Zambon, and Insmed Incorporated; a grant from Gilead; and personal fees from Novartis and Chiesi.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

