Immunogenicity evaluation of ChAdox1 nCov-19 (AZD1222) vaccine in solid cancer patients in Chulabhorn Hospital
- PMID: 35976687
- PMCID: PMC9746434
- DOI: 10.1080/21645515.2022.2104058
Immunogenicity evaluation of ChAdox1 nCov-19 (AZD1222) vaccine in solid cancer patients in Chulabhorn Hospital
Abstract
Introduction: Cancer patients are more vulnerable to coronavirus disease 2019 (COVID-19) owing to their compromised immune status. However, data regarding COVID-19 vaccine safety and immune response in cancer patients are scarce.
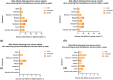
Method: This prospective, age- and sex-matched, single-center cohort study included 61 cancer patients and 122 healthy control participants. Seropositivity was defined as anti-S IgG titer >0.8 units/ml. Primary end point was seroconversion rate of immunoglobulin (Ig)G antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein (anti-S IgG) in cancer patients vs. healthy control participants following the second dose of COVID-19 vaccine ChAdOx1 nCoV-19 (AZD1222).
Results: After the second-dose vaccination, there was no difference in seropositivity rate between groups (57 [93.44%] patients with cancer vs. 121 [99.18%] control participants; geometric mean ratio [GMR]: 0.39; 95%CI: 0.01-10.46; p-value = 0.571). In contrast, after the first-dose vaccination, the seropositivity rate was significantly lower in the cancer patients than in the control participants (50/61 [81.97%] vs. 121/122 [99.18%]; GMR: 0.07; 95%CI: 0.01-0.71; p = 0.025). The median anti-S IgG titer after the first-and second dose vaccination were not significantly different between groups. Female sex was significantly associated with a higher anti-S IgG titer. 5FU- and taxane-based chemotherapy regimens were associated with a lower IgG titer. Side effects of vaccination were tolerable.
Conclusions: The anti-S IgG seropositivity rate after completing the second vaccine dose did not differ between the cancer patients and control participants. However, the anti-S IgG seropositivity rate after the first-dose vaccination was lower in cancer patients.
Keywords: AZD1222; ChAdox1 nCov-19; seropositivity; solid cancer; titer; vaccine.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- WHO Coronavirus (COVID-19) Dashboard: 263,563,622 confirmed cases and 5,232,562 deaths from COVID-19 pandemic [Internet]. [accessed 2022 Apr 8]. https://covid19.who.int/.
-
- Pathania AS, Prathipati P, Abdul BA, Chava S, Katta SS, Gupta SC, Gangula PR, Pandey MK, Durden DL, Byrareddy SN et al. COVID-19 and cancer comorbidity: therapeutic opportunities and challenges. Theranostics. 2021;11(2):1–8. PMID: 33391502; PMCID: PMC7738845. doi:10.7150/thno.51471. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous