Regulation of membrane scission in yeast endocytosis
- PMID: 35976707
- PMCID: PMC9635293
- DOI: 10.1091/mbc.E21-07-0346
Regulation of membrane scission in yeast endocytosis
Abstract
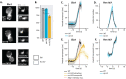
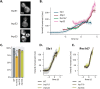
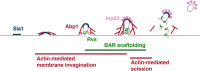
During clathrin-mediated endocytosis, a flat plasma membrane is shaped into an invagination that undergoes scission to form a vesicle. In mammalian cells, the force that drives the transition from invagination to vesicle is primarily provided by the GTPase dynamin that acts in concert with crescent-shaped BAR domain proteins. In yeast cells, the mechanism of endocytic scission is unclear. The yeast BAR domain protein complex Rvs161/167 (Rvs) nevertheless plays an important role in this process: deletion of Rvs dramatically reduces scission efficiency. A mechanistic understanding of the influence of Rvs on scission, however, remains incomplete. We used quantitative live-cell imaging and genetic manipulation to understand the recruitment and function of Rvs and other late-stage proteins at yeast endocytic sites. We found that arrival of Rvs at endocytic sites is timed by interaction of its BAR domain with specific membrane curvature. A second domain of Rvs167-the SH3 domain-affects localization efficiency of Rvs. We show that Myo3, one of the two type-I myosins in Saccharomyces cerevisiae, has a role in recruiting Rvs167 via the SH3 domain. Removal of the SH3 domain also affects assembly and disassembly of actin and impedes membrane invagination. Our results indicate that both BAR and SH3 domains are important for the role of Rvs as a regulator of scission. We tested other proteins implicated in vesicle formation in S. cerevisiae and found that neither synaptojanins nor dynamin contribute directly to membrane scission. We propose that recruitment of Rvs BAR domains delays scission and allows invaginations to grow by stabilizing them. We also propose that vesicle formation is dependent on the force exerted by the actin network.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

