Resistance levels to non-nucleoside reverse transcriptase inhibitors among pregnant women with recent HIV infection in Malawi
- PMID: 35976773
- PMCID: PMC9555317
- DOI: 10.1177/13596535221121225
Resistance levels to non-nucleoside reverse transcriptase inhibitors among pregnant women with recent HIV infection in Malawi
Abstract
Background: Information on HIV drug resistance (HIVDR) prevalence in people newly diagnosed with HIV is limited. We implemented a cross-sectional study to estimate HIVDR prevalence among pregnant women recently infected with HIV in Malawi.
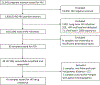
Methods: The HIVDR study was nested within a routine antenatal clinic (ANC) sentinel surveillance survey. Dried blood spot samples were tested for recent infection using a limiting antigen antibody assay together with HIV viral load testing. HIV-1 protease and reverse transcriptase were sequenced using Sanger sequencing. Drug susceptibility was predicted using Stanford HIVdb algorithm (version 8.9). Weighted analysis was performed in Stata 15.1.
Results: Of the 21,642 pregnant women enrolled in the ANC survey, 8.4% (1826/21,642) tested HIV positive. Of these, 5.0% (92/1826) had recent HIV infection, and 90.2% (83/92) were tested by PCR. The amplification and sequencing success rate was 57.8% (48/83). The prevalence of any HIVDR was 14.6% (5/45) (95% CI: 4.7-36.8%), all of which indicated HIVDR to nonnucleoside reverse transcriptase inhibitors (NNRTIs). HIVDR to nucleoside reverse transcriptase inhibitors was 7.9% (2/45) (95% CI: 1.4-34.6%). Resistance to protease inhibitors currently in use in Malawi was not observed.
Conclusions: Despite the low number of cases with presumed TDR, our study hints that resistance to NNRTIs was high, above the 10% target for regimen change. Further investigation is needed to establish the exact magnitude of presumed TDR among women recently infected with HIV. These findings support the transition to an integrase inhibitor-based first-line regimen for patients initiating or on ART.
Conflict of interest statement
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
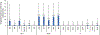
References
-
- Boerma RS, Sigaloff KC, Akanmu AS, et al. Alarming increase in pretreatment HIV drug resistance in children living in sub-Saharan Africa: a systematic review and meta-analysis. J Antimicrob Chemother 2017; 72(2): 365–371. - PubMed
-
- Avila-Rios S, Garcia-Morales C, Matias-Florentino M, et al. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: a nationally representative 2015 WHO survey. Lancet HIV 2016; 3(12):e579–e591. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

