High-efficiency quantitative control of mitochondrial transfer based on droplet microfluidics and its application on muscle regeneration
- PMID: 35977014
- PMCID: PMC9385153
- DOI: 10.1126/sciadv.abp9245
High-efficiency quantitative control of mitochondrial transfer based on droplet microfluidics and its application on muscle regeneration
Abstract
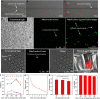
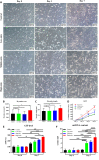
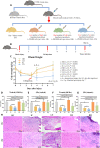
Mitochondrial transfer is a spontaneous process to restore damaged cells in various pathological conditions. The transfer of mitochondria to cell therapy products before their administration can enhance therapeutic outcomes. However, the low efficiency of previously reported methods limits their clinical application. Here, we developed a droplet microfluidics-based mitochondrial transfer technique that can achieve high-efficiency and high-throughput quantitative mitochondrial transfer to single cells. Because mitochondria are essential for muscles, myoblast cells and a muscle injury model were used as a proof-of-concept model to evaluate the proposed technique. In vitro and in vivo experiments demonstrated that C2C12 cells with 31 transferred mitochondria had significant improvements in cellular functions compared to those with 0, 8, and 14 transferred mitochondria and also had better therapeutic effects on muscle regeneration. The proposed technique can considerably promote the clinical application of mitochondrial transfer, with optimized cell function improvements, for the cell therapy of mitochondria-related diseases.
Figures




References
-
- Picard M., Zhang J., Hancock S., Derbeneva O., Golhar R., Golik P., O’Hearn S., Levy S., Potluri P., Lvova M., Davila A., Lin C. S., Perin J. C., Rappaport E. F., Hakonarson H., Trounce I. A., Procaccio V., Wallace D. C., Progressive increase in mtDNA 3243A > G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl. Acad. Sci. U.S.A. 111, E4033–E4042 (2014). - PMC - PubMed
LinkOut - more resources
Full Text Sources

