An epidermal electronic system for physiological information acquisition, processing, and storage with an integrated flash memory array
- PMID: 35977018
- PMCID: PMC9385141
- DOI: 10.1126/sciadv.abp8075
An epidermal electronic system for physiological information acquisition, processing, and storage with an integrated flash memory array
Abstract
Epidermal electronic systems that simultaneously provide physiological information acquisition, processing, and storage are in high demand for health care/clinical applications. However, these system-level demonstrations using flexible devices are still challenging because of obstacles in device performance, functional module construction, or integration scale. Here, on the basis of carbon nanotubes, we present an epidermal system that incorporates flexible sensors, sensor interface circuits, and an integrated flash memory array to collect physiological information from the human body surface; amplify weak biosignals by high-performance differential amplifiers (voltage gain of 27 decibels, common-mode rejection ratio of >43 decibels, and gain bandwidth product of >22 kilohertz); and store the processed information in the memory array with performance on par with industrial standards (retention time of 108 seconds, program/erase voltages of ±2 volts, and endurance of 106 cycles). The results shed light on the great application potential of epidermal electronic systems in personalized diagnostic and physiological monitoring.
Figures
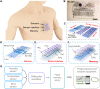
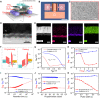



References
-
- Kim D.-H., Lu N., Ma R., Kim Y.-S., Kim R.-H., Wang S., Wu J., Won S. M., Tao H., Islam A., Yu K. J., Kim T.-I., Chowdhury R., Ying M., Xu L., Li M., Chung H.-J., Keum H., McCormick M., Liu P., Zhang Y.-W., Omenetto F. G., Huang Y., Coleman T., Rogers J. A., Epidermal electronics. Science 333, 838–843 (2011). - PubMed
-
- Xiang L., Zeng X., Xia F., Jin W., Liu Y., Hu Y., Recent advances in flexible and stretchable sensing systems: From the perspective of system integration. ACS Nano 14, 6449–6469 (2020). - PubMed
-
- Ray T. R., Choi J., Bandodkar A. J., Krishnan S., Gutruf P., Tian L., Ghaffari R., Rogers J. A., Bio-integrated wearable systems: A comprehensive review. Chem. Rev. 119, 5461–5533 (2019). - PubMed
-
- Liu Y., Pharr M., Salvatore G. A., Lab-on-Skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 11, 9614–9635 (2017). - PubMed
-
- Lim H.-R., Kim H. S., Qazi R., Kwon Y.-T., Jeong J.-W., Yeo W.-H., Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv. Mater. 32, 1901924 (2020). - PubMed
LinkOut - more resources
Full Text Sources

