A dysregulated sebum-microbial metabolite-IL-33 axis initiates skin inflammation in atopic dermatitis
- PMID: 35977109
- PMCID: PMC9375142
- DOI: 10.1084/jem.20212397
A dysregulated sebum-microbial metabolite-IL-33 axis initiates skin inflammation in atopic dermatitis
Abstract
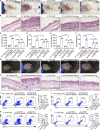
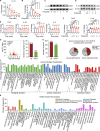
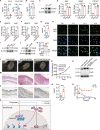
Microbial dysbiosis in the skin has been implicated in the pathogenesis of atopic dermatitis (AD); however, whether and how changes in the skin microbiome initiate skin inflammation, or vice versa, remains poorly understood. Here, we report that the levels of sebum and its microbial metabolite, propionate, were lower on the skin surface of AD patients compared with those of healthy individuals. Topical propionate application attenuated skin inflammation in mice with MC903-induced AD-like dermatitis by inhibiting IL-33 production in keratinocytes, an effect that was mediated through inhibition of HDAC and regulation of the AhR signaling pathway. Mice lacking sebum spontaneously developed AD-like dermatitis, which was improved by topical propionate application. A proof-of-concept clinical study further demonstrated the beneficial therapeutic effects of topical propionate application in AD patients. In summary, we have uncovered that the dysregulated sebum-microbial metabolite-IL-33 axis might play an initiating role in AD-related skin inflammation, thereby highlighting novel therapeutic strategies for the treatment of AD.
© 2022 Qiu et al.
Conflict of interest statement
Disclosures: The authors declare no competing financial interests.
Figures










References
-
- Baurecht, H., Rühlemann M.C., Rodríguez E., Thielking F., Harder I., Erkens A.-S., Stölzl D., Ellinghaus E., Hotze M., Lieb W., et al. . 2018. Epidermal lipid composition, barrier integrity, and eczematous inflammation are associated with skin microbiome configuration. J. Allergy Clin. Immunol. 141:1668–1676.e16. 10.1016/j.jaci.2018.01.019 - DOI - PubMed
-
- Bissonnette, R., Risch J.E., Mcelwee K.J., Marchessault P., Bolduc C., Nigen S., and Maari C.. 2015. Changes in serum free testosterone, sleep patterns, and 5-α-reductase type I activity influence changes in sebum excretion in female subjects. Skin Res. Technol. 21:47–53. 10.1111/srt.12155 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2021-01-07-00-07-E00078/Key Project of the Innovation Program of Shanghai Municipal Education Commission
- 81972939/National Natural Science Foundation of China
- Milstein Medical Asian American Partnership Foundation
- 2019060001/Nanjing Incubation Program for National Clinical Research Centre
- BE2020632/Key Project of Social Development in Jiangsu Province

