Association of Medicaid Managed Care Drug Carve Outs With Hepatitis C Virus Prescription Use
- PMID: 35977199
- PMCID: PMC8796891
- DOI: 10.1001/jamahealthforum.2021.2285
Association of Medicaid Managed Care Drug Carve Outs With Hepatitis C Virus Prescription Use
Abstract
Importance: Medicaid enrolls a disproportionate share of US adults with hepatitis C virus (HCV), and most receive Medicaid benefits through managed care organizations (MCOs). Medicaid MCOs often impose stricter requirements to access HCV medications than traditional fee-for-service Medicaid, which may inhibit use. Though Medicaid MCOs generally cover prescription drugs, several states have carved out direct-acting antiviral HCV medications from MCO coverage and opted to cover them under fee-for-service. Whether these carve outs were associated with changes in medication use is unknown.
Objective: To examine the association between Medicaid-covered HCV medication fills and carve outs of these medications from MCO coverage.
Design setting and participants: This cross-sectional study examined changes in fills of Medicaid-covered direct-acting antiviral HCV medications in 4 states (Indiana, Michigan, New Hampshire, and West Virginia) that carved out these drugs from Medicaid MCOs between 2015 and 2017. A synthetic control approach was used to compare changes in HCV prescription fills between states that did and did not carve out these medications from MCO prescription drug coverage. Data of direct-acting antiviral HCV prescription fills were obtained from the Medicaid State Drug Utilization Data files, January 2015 to June 2020. Data analysis was conducted from November 2020 to June 2021.
Exposures: Carve outs of direct-acting antiviral HCV medications from Medicaid MCO prescription drug coverage.
Main outcomes and measures: Direct-acting antiviral HCV prescriptions filled per 100 000 Medicaid enrollees.
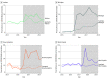
Results: In this cross-sectional study, carve outs were associated with a mean quarterly increase of 22.1 (95% CI, 12.7-34.1) HCV prescriptions per 100 000 Medicaid enrollees, a relative increase of 86.3% compared with synthetic control states. Compared with each state's respective synthetic control, HCV prescription fills were associated with an increase of 11.5 (95% CI, 5.1-19.0) HCV prescription fills per 100 000 Medicaid enrollees per quarter in Indiana, 36.6 (95% CI, 23.5-53.9) in Michigan, 20.7 (95% CI, 11.1-32.8) in West Virginia, and 43.6 (95% CI, 25.9-68.4) in New Hampshire.
Conclusions and relevance: In this cross-sectional study of data from 39 states and the District of Columbia, carve outs of direct-acting antiviral HCV medications from Medicaid MCO prescription drug coverage were associated with significant increases in HCV medication use. Given their clinical benefits, greater uptake of HCV medication may help improve the health of Medicaid enrollees with HCV and reduce the economic burden of untreated HCV on the US health care system.
Copyright 2021 Auty SG et al. JAMA Health Forum.
Conflict of interest statement
Conflict of Interest Disclosures: Ms Auty is separately supported by the National Institute of Drug Abuse (T32-DA041898-03) and Dr Griffith is separately supported by the Agency for Healthcare Research and Quality (K12-HS026395). Dr Shafer reported receiving research support for unrelated work from the Robert Wood Johnson Foundation, the Commonwealth Fund, Renova Health, and the Department of Veterans Affairs (through a contract with the Boston University School of Public Health) and serving as a consultant to Patient Funding Alternatives for unrelated work. Dr Dusetzina reported grants from the Robert Wood Johnson Foundation, grants from The Leukemia & Lymphoma Society, grants from Arnold Ventures, personal fees from West Health, personal fees from the National Academy of State Health Policy, and personal fees from ICER outside the submitted work. No other disclosures were reported.
Figures
Comment in
- doi: 10.1001/jamahealthforum.2021.2412
Similar articles
-
Medicaid Subscription-Based Payment Models and Implications for Access to Hepatitis C Medications.JAMA Health Forum. 2021 Aug 27;2(8):e212291. doi: 10.1001/jamahealthforum.2021.2291. eCollection 2021 Aug. JAMA Health Forum. 2021. PMID: 35977192 Free PMC article.
-
Substance use disorder treatment carve outs in Medicaid managed care.J Subst Use Addict Treat. 2024 Jun;161:209357. doi: 10.1016/j.josat.2024.209357. Epub 2024 Mar 28. J Subst Use Addict Treat. 2024. PMID: 38554998 Free PMC article.
-
Association Between Medicaid Managed Care Coverage of Substance Use Services and Treatment Utilization.JAMA Health Forum. 2022 Aug 5;3(8):e222812. doi: 10.1001/jamahealthforum.2022.2812. JAMA Health Forum. 2022. PMID: 36218990 Free PMC article.
-
Medicaid and Medicare Utilization of Direct-Acting Antiviral Medications for Patients With Hepatitis C.Gastro Hep Adv. 2024 Nov 4;4(3):100584. doi: 10.1016/j.gastha.2024.10.024. eCollection 2025. Gastro Hep Adv. 2024. PMID: 39931049 Free PMC article. Review. No abstract available.
-
Rationing Care: Barriers to Direct-Acting Antiviral Treatment in Medicaid Treatment Criteria.Clin Liver Dis (Hoboken). 2018 Dec 14;12(5):122-124. doi: 10.1002/cld.751. eCollection 2018 Nov. Clin Liver Dis (Hoboken). 2018. PMID: 30988926 Free PMC article. Review. No abstract available.
Cited by
-
Anticipated efficiencies, real costs: Medicaid managed care organizations and the pharmacy benefit.J Manag Care Spec Pharm. 2022 Mar;28(3):354-361. doi: 10.18553/jmcp.2022.28.3.354. J Manag Care Spec Pharm. 2022. PMID: 35199580 Free PMC article. Review.
-
Medicaid Policy and Hepatitis C Treatment Among Rural People Who Use Drugs.Med Care. 2025 Feb 1;63(2):77-88. doi: 10.1097/MLR.0000000000002095. Epub 2024 Nov 18. Med Care. 2025. PMID: 39791842
-
Hepatitis C Care in the Greater New Orleans Area: Patient Perspectives on the Barriers and Facilitators to Care.J Health Care Poor Underserved. 2025;36(1):257-283. doi: 10.1353/hpu.2025.a951596. J Health Care Poor Underserved. 2025. PMID: 39957649 Free PMC article.
-
Prevalence of Testing for Human Immunodeficiency Virus, Hepatitis B Virus, and Hepatitis C Virus Among Medicaid Enrollees Treated With Medications for Opioid Use Disorder in 11 States, 2016-2019.Clin Infect Dis. 2023 May 24;76(10):1793-1801. doi: 10.1093/cid/ciac981. Clin Infect Dis. 2023. PMID: 36594172 Free PMC article.
-
Changes in Use of Hepatitis C Direct-Acting Antivirals After Access Restrictions Were Eased by State Medicaid Programs.JAMA Health Forum. 2024 Apr 5;5(4):e240302. doi: 10.1001/jamahealthforum.2024.0302. JAMA Health Forum. 2024. PMID: 38578628 Free PMC article.
References
-
- Younossi Z, Gordon SC, Ahmed A, Dieterich D, Saab S, Beckerman R. Treating Medicaid patients with hepatitis C: clinical and economic impact. Am J Manag Care. 2017;23(2):107-112. - PubMed
-
- Roebuck MC, Liberman JN. Assessing the burden of illness of chronic hepatitis C and impact of direct-acting antiviral use on healthcare costs in Medicaid. Am J Manag Care. 2019;25(8)(suppl):S131-S139. - PubMed

