Buprenorphine Use Trends Following Removal of Prior Authorization Policies for the Treatment of Opioid Use Disorder in 2 State Medicaid Programs
- PMID: 35977240
- PMCID: PMC9233239
- DOI: 10.1001/jamahealthforum.2022.1757
Buprenorphine Use Trends Following Removal of Prior Authorization Policies for the Treatment of Opioid Use Disorder in 2 State Medicaid Programs
Abstract
Importance: State Medicaid programs have implemented initiatives to expand treatment coverage for opioid use disorder (OUD); however, some Medicaid programs still require prior authorizations (PAs) for filling buprenorphine prescriptions.
Objective: To evaluate the changes in buprenorphine use for OUD among Medicaid enrollees in states that completely removed buprenorphine PA requirements.
Design setting and participants: This retrospective cross-sectional study analyzed the immediate and trend changes on buprenorphine use during 2013 to 2020 associated with removal of PA requirements using a controlled interrupted time series analysis to account for autocorrelation. Data were collected from Medicaid State Drug Utilization Data for 2 states (California and Illinois) that completely removed a buprenorphine PA during the study period, and buprenorphine prescriptions for OUD treatment were identified among Medicaid enrollees.
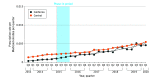
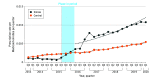
Main outcomes and measures: Quarterly total number of buprenorphine prescriptions for each state was calculated, and stratification analyses were conducted by dosage form (films and tablets).
Results: Among the 2 state Medicaid programs (California and Illinois) that removed buprenorphine PAs, there was a total of 702 643 and 415 115 eligible buprenorphine prescription claims, respectively. After removing PA requirements for buprenorphine, there was an immediate increase that was not statistically significant (rate ratio [RR], 1.11; 95% CI, 0.76-1.61) in the number of all buprenorphine prescriptions in California and a statistically significant increase (RR, 6.99; 95% CI, 4.67-10.47) in the number of all buprenorphine prescriptions in Illinois relative to the change in the control states (Alabama, Florida, Idaho, Kansas, Mississippi, Nevada, South Dakota, and Wyoming). Additionally, there was a statistically significant decreasing trend in the number of all buprenorphine prescriptions in California (RR, 0.88; 95% CI, 0.82-0.94) and a statistically significant increasing trend in Illinois (RR, 1.11; 95% CI, 1.05-1.19) relative to the trend in control states.
Conclusions and relevance: In this cross-sectional study, removal of buprenorphine PA requirements was associated with a statistically significant increase in the number of buprenorphine prescription fills among Medicaid populations in 1 of the 2 included states.
Copyright 2022 Keshwani S et al. JAMA Health Forum.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Lo-Ciganic reported grants from the National Institute on Drug Abuse, the National Institute on Aging, the National Institute of Mental Health, Merck Sharp & Dohme, Bristol Myers Squibb, the Richard King Mellon Foundation at the University of Pittsburgh, the Clinical and Translational Science Institute of the University of Florida, the Pharmaceutical Research and Manufacturers of America (PhRMA) Foundation, and the US Department of Veterans Affairs outside the submitted work; in addition, Dr Lo-Ciganic has a patent for U1195.70174US00 pending. Dr Wilson reported grants from the National Institute on Drug Abuse, the National Institute on Aging, Merck Sharp & Dohme, and Bristol Myers Squibb outside the submitted work, as well as serving as an editorial board member for the Journal of Pharmacy Technology. Dr Hincapie-Castillo reported grants from Merck outside the submitted work. No other disclosures were reported.
Figures

Comment in
-
The Consequences of Removing Prior Authorization for Buprenorphine in Medicaid-Building an Evidence Base.JAMA Health Forum. 2022 Jun 3;3(6):e220189. doi: 10.1001/jamahealthforum.2022.0189. JAMA Health Forum. 2022. PMID: 36219020 No abstract available.
References
-
- 2019. National Survey on Drug Use and Health: detailed tables. Substance Abuse and Mental Health Services Administration . September 11, 2020. Accessed May 19, 2022. https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables
-
- Meges D, Bascelli L, Bhalla P, et al. Adapting your practice: recommendations for the care of homeless patients with opioid use disorders. Health Care for the Homeless Clinicians’ Network . March 2014. Accessed May 23, 2022. https://opioidresponsenetwork.org/ResourceMaterials/hch-opioid-use-disor...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

