M6L12 Nanospheres with Multiple C70 Binding Sites for 1O2 Formation in Organic and Aqueous Media
- PMID: 35977385
- PMCID: PMC9437924
- DOI: 10.1021/jacs.2c05507
M6L12 Nanospheres with Multiple C70 Binding Sites for 1O2 Formation in Organic and Aqueous Media
Abstract
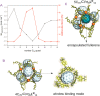
Singlet oxygen is a potent oxidant with major applications in organic synthesis and medicinal treatment. An efficient way to produce singlet oxygen is the photochemical generation by fullerenes which exhibit ideal thermal and photochemical stability. In this contribution we describe readily accessible M6L12 nanospheres with unique binding sites for fullerenes located at the windows of the nanospheres. Up to four C70 can be associated with a single nanosphere, presenting an efficient method for fullerene extraction and application. Depending on the functionality located on the outside of the sphere, they act as vehicles for 1O2 generation in organic or in aqueous media using white LED light. Excellent productivity in 1O2 generation and consecutive oxidation of 1O2 acceptors using C70⊂[Pd6L12], C60⊂[Pd6L12] or fullerene soot extract was observed. The methodological design principles allow preparation and application of highly effective multifullerene binding spheres.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

