hiPSC-derived bone marrow milieu identifies a clinically actionable driver of niche-mediated treatment resistance in leukemia
- PMID: 35977468
- PMCID: PMC9418860
- DOI: 10.1016/j.xcrm.2022.100717
hiPSC-derived bone marrow milieu identifies a clinically actionable driver of niche-mediated treatment resistance in leukemia
Abstract
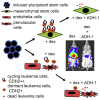
Leukemia cells re-program their microenvironment to augment blast proliferation and enhance treatment resistance. Means of clinically targeting such niche-driven treatment resistance remain ambiguous. We develop human induced pluripotent stem cell (hiPSC)-engineered niches to reveal druggable cancer-niche dependencies. We reveal that mesenchymal (iMSC) and vascular niche-like (iANG) hiPSC-derived cells support ex vivo proliferation of patient-derived leukemia cells, affect dormancy, and mediate treatment resistance. iMSCs protect dormant and cycling blasts against dexamethasone, while iANGs protect only dormant blasts. Leukemia proliferation and protection from dexamethasone-induced apoptosis is dependent on cancer-niche interactions mediated by CDH2. Consequently, we test CDH2 antagonist ADH-1 (previously in Phase I/II trials for solid tumors) in a very aggressive patient-derived xenograft leukemia mouse model. ADH-1 shows high in vivo efficacy; ADH-1/dexamethasone combination is superior to dexamethasone alone, with no ADH-1-conferred additional toxicity. These findings provide a proof-of-concept starting point to develop improved, potentially safer therapeutics targeting niche-mediated cancer dependencies in blood cancers.
Keywords: cancer microenvironment; dormancy; drugging cancer niche; iPSC-niche; treatment resistance.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Patient-Derived Bone Marrow Spheroids Reveal Leukemia-Initiating Cells Supported by Mesenchymal Hypoxic Niches in Pediatric B-ALL.Front Immunol. 2021 Oct 19;12:746492. doi: 10.3389/fimmu.2021.746492. eCollection 2021. Front Immunol. 2021. PMID: 34737747 Free PMC article.
-
Human bone marrow niche chemoprotection mediated by cytochrome P450 enzymes.Oncotarget. 2015 Jun 20;6(17):14905-12. doi: 10.18632/oncotarget.3614. Oncotarget. 2015. PMID: 25915157 Free PMC article.
-
Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia.Hematology. 2018 Dec;23(10):729-739. doi: 10.1080/10245332.2018.1486064. Epub 2018 Jun 14. Hematology. 2018. PMID: 29902132 Review.
-
iPSC-Derived Hereditary Breast Cancer Model Reveals the BRCA1-Deleted Tumor Niche as a New Culprit in Disease Progression.Int J Mol Sci. 2021 Jan 27;22(3):1227. doi: 10.3390/ijms22031227. Int J Mol Sci. 2021. PMID: 33513753 Free PMC article.
-
The hematopoietic stem-cell niche in health and leukemia.Cell Mol Life Sci. 2017 Feb;74(4):579-590. doi: 10.1007/s00018-016-2306-y. Epub 2016 Jul 19. Cell Mol Life Sci. 2017. PMID: 27436341 Free PMC article. Review.
Cited by
-
The role of microenvironment in the initiation and evolution of B-cell precursor acute lymphoblastic leukemia.Front Oncol. 2023 Mar 7;13:1150612. doi: 10.3389/fonc.2023.1150612. eCollection 2023. Front Oncol. 2023. PMID: 36959797 Free PMC article. Review.
-
Advancements and Innovative Strategies in Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cell Therapy: A Comprehensive Review.Stem Cells Int. 2024 Sep 30;2024:4073485. doi: 10.1155/2024/4073485. eCollection 2024. Stem Cells Int. 2024. PMID: 39377039 Free PMC article. Review.
-
Targeting N-cadherin (CDH2) and the malignant bone marrow microenvironment in acute leukaemia.Expert Rev Mol Med. 2023 May 3;25:e16. doi: 10.1017/erm.2023.13. Expert Rev Mol Med. 2023. PMID: 37132370 Free PMC article. Review.
-
Epithelial-Mesenchymal Transition in Acute Leukemias.Int J Mol Sci. 2024 Feb 11;25(4):2173. doi: 10.3390/ijms25042173. Int J Mol Sci. 2024. PMID: 38396852 Free PMC article. Review.
-
Distinct stromal cell populations define the B-cell acute lymphoblastic leukemia microenvironment.Leukemia. 2025 Aug 15. doi: 10.1038/s41375-025-02734-z. Online ahead of print. Leukemia. 2025. PMID: 40817406
References
-
- Agarwal P., Isringhausen S., Li H., Paterson A.J., He J., Gomariz Á., Nagasawa T., Nombela-Arrieta C., Bhatia R. Mesenchymal niche-specific expression of Cxcl12 controls quiescence of treatment-resistant leukemia stem cells. Cell Stem Cell. 2019;24:769–784.e6. doi: 10.1016/j.stem.2019.02.018. - DOI - PMC - PubMed
-
- Zhang B., Nguyen L.X.T., Li L., Zhao D., Kumar B., Wu H., Lin A., Pellicano F., Hopcroft L., Su Y.L., et al. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat. Med. 2018;24:450–462. doi: 10.1038/nm.4499. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

