Stromal remodeling regulates dendritic cell abundance and activity in the tumor microenvironment
- PMID: 35977482
- PMCID: PMC9402878
- DOI: 10.1016/j.celrep.2022.111201
Stromal remodeling regulates dendritic cell abundance and activity in the tumor microenvironment
Abstract
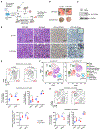
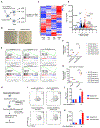
Stimulatory type 1 conventional dendritic cells (cDC1s) engage in productive interactions with CD8+ effectors along tumor-stroma boundaries. The paradoxical accumulation of "poised" cDC1s within stromal sheets is unlikely to simply reflect passive exclusion from tumor cores. Drawing parallels with embryonic morphogenesis, we hypothesized that invasive margin stromal remodeling generates developmentally conserved cell fate cues that regulate cDC1 behavior. We find that, in human T cell-inflamed tumors, CD8+ T cells penetrate tumor nests, whereas cDC1s are confined within adjacent stroma that recurrently displays site-specific proteolysis of the matrix proteoglycan versican (VCAN), an essential organ-sculpting modification in development. VCAN is necessary, and its proteolytic fragment (matrikine) versikine is sufficient for cDC1 accumulation. Versikine does not influence tumor-seeding pre-DC differentiation; rather, it orchestrates a distinctive cDC1 activation program conferring exquisite sensitivity to DNA sensing, supported by atypical innate lymphoid cells. Thus, peritumoral stroma mimicking embryonic provisional matrix remodeling regulates cDC1 abundance and activity to elicit T cell-inflamed tumor microenvironments.
Keywords: CD40; CP: Cancer; CP: Immunology; cDC1; checkpoint inhibitors; dendritic cells; immunotherapy; proteoglycans; tumor matrix; tumor stroma; versican; versikine.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests F.A. and C.H. are listed as inventors on US patent US20170258898A1: “Versikine for inducing or potentiating an immune response.”
Figures





References
-
- Balan S, Radford KJ, and Bhardwaj N (2020). Unexplored horizons of cDC1 in immunity and tolerance. Adv. Immunol 148, 49–91. - PubMed
-
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, and Banchereau J (1999). In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med 190, 1417–1426. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

