A cytoskeletal vortex drives phage nucleus rotation during jumbo phage replication in E. coli
- PMID: 35977483
- PMCID: PMC9891218
- DOI: 10.1016/j.celrep.2022.111179
A cytoskeletal vortex drives phage nucleus rotation during jumbo phage replication in E. coli
Abstract
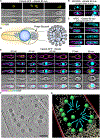
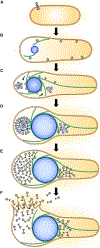
Nucleus-forming jumbo phages establish an intricate subcellular organization, enclosing phage genomes within a proteinaceous shell called the phage nucleus. During infection in Pseudomonas, some jumbo phages assemble a bipolar spindle of tubulin-like PhuZ filaments that positions the phage nucleus at midcell and drives its intracellular rotation. This facilitates the distribution of capsids on its surface for genome packaging. Here we show that the Escherichia coli jumbo phage Goslar assembles a phage nucleus surrounded by an array of PhuZ filaments resembling a vortex instead of a bipolar spindle. Expression of a mutant PhuZ protein strongly reduces Goslar phage nucleus rotation, demonstrating that the PhuZ cytoskeletal vortex is necessary for rotating the phage nucleus. While vortex-like cytoskeletal arrays are important in eukaryotes for cytoplasmic streaming and nucleus alignment, this work identifies a coherent assembly of filaments into a vortex-like structure driving intracellular rotation within the prokaryotic cytoplasm.
Keywords: CP: Microbiology; bacterial cell biology; bacterial cytoskeleton; bacteriophage; bacteriophage replication; phage nucleus; phage tubulin.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Similar articles
-
Erwinia phage Asesino is a nucleus-forming phage that lacks PhuZ.Sci Rep. 2025 Jan 11;15(1):1692. doi: 10.1038/s41598-024-64095-4. Sci Rep. 2025. PMID: 39799172 Free PMC article.
-
The Phage Nucleus and PhuZ Spindle: Defining Features of the Subcellular Organization and Speciation of Nucleus-Forming Jumbo Phages.Front Microbiol. 2021 Jul 13;12:641317. doi: 10.3389/fmicb.2021.641317. eCollection 2021. Front Microbiol. 2021. PMID: 34326818 Free PMC article. Review.
-
Intracellular Organization by Jumbo Bacteriophages.J Bacteriol. 2020 Dec 18;203(2):e00362-20. doi: 10.1128/JB.00362-20. Print 2020 Dec 18. J Bacteriol. 2020. PMID: 32868402 Free PMC article. Review.
-
The Phage Nucleus and Tubulin Spindle Are Conserved among Large Pseudomonas Phages.Cell Rep. 2017 Aug 15;20(7):1563-1571. doi: 10.1016/j.celrep.2017.07.064. Cell Rep. 2017. PMID: 28813669 Free PMC article.
-
Viral Capsid Trafficking along Treadmilling Tubulin Filaments in Bacteria.Cell. 2019 Jun 13;177(7):1771-1780.e12. doi: 10.1016/j.cell.2019.05.032. Cell. 2019. PMID: 31199917 Free PMC article.
Cited by
-
Erwinia phage Asesino is a nucleus-forming phage that lacks PhuZ.Sci Rep. 2025 Jan 11;15(1):1692. doi: 10.1038/s41598-024-64095-4. Sci Rep. 2025. PMID: 39799172 Free PMC article.
-
Sequential membrane- and protein-bound organelles compartmentalize genomes during phage infection.Cell Host Microbe. 2025 Apr 9;33(4):484-497.e6. doi: 10.1016/j.chom.2025.03.005. Epub 2025 Mar 31. Cell Host Microbe. 2025. PMID: 40168997
-
Identification of the bacteriophage nucleus protein interaction network.bioRxiv [Preprint]. 2023 May 18:2023.05.18.541317. doi: 10.1101/2023.05.18.541317. bioRxiv. 2023. Update in: Nat Struct Mol Biol. 2023 Nov;30(11):1653-1662. doi: 10.1038/s41594-023-01094-5. PMID: 37292858 Free PMC article. Updated. Preprint.
-
A flagella-dependent Burkholderia jumbo phage controls rice seedling rot and steers Burkholderia glumae toward reduced virulence in rice seedlings.mBio. 2025 Mar 12;16(3):e0281424. doi: 10.1128/mbio.02814-24. Epub 2025 Jan 27. mBio. 2025. PMID: 39868782 Free PMC article.
-
The intricate organizational strategy of nucleus-forming phages.Curr Opin Microbiol. 2024 Jun;79:102457. doi: 10.1016/j.mib.2024.102457. Epub 2024 Apr 6. Curr Opin Microbiol. 2024. PMID: 38581914 Free PMC article. Review.
References
-
- Burt A, Gaifas L, Dendooven T, and Gutsche I. (2021). Tools enabling flexible approaches to high-resolution subtomogram averaging. Preprint at bio-Rxiv. 10.1101/2021.01.31.428990. - DOI
-
- Castaño-Díez D, Kudryashev M, Arheit M, and Stahlberg H. (2012). Dynamo: a flexible, user-friendly development tool for subtomogram averaging of cryo-EM data in high-performance computing environments. J. Struct. Biol 178, 139–151. - PubMed
-
- Castaño-Díez D, Kudryashev M, and Stahlberg H. (2017). Dynamo Catalogue: geometrical tools and data management for particle picking in subtomogram averaging of cryo-electron tomograms. J. Struct. Biol 197, 135–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

