Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma
- PMID: 35977543
- PMCID: PMC9488669
- DOI: 10.1016/j.immuni.2022.07.014
Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma
Abstract
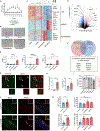
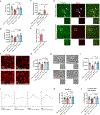
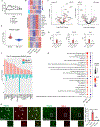
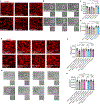
The apolipoprotein E4 (APOE4) allele is associated with an increased risk of Alzheimer disease and a decreased risk of glaucoma, but the underlying mechanisms remain poorly understood. Here, we found that in two mouse glaucoma models, microglia transitioned to a neurodegenerative phenotype characterized by upregulation of Apoe and Lgals3 (Galectin-3), which were also upregulated in human glaucomatous retinas. Mice with targeted deletion of Apoe in microglia or carrying the human APOE4 allele were protected from retinal ganglion cell (RGC) loss, despite elevated intraocular pressure (IOP). Similarly to Apoe-/- retinal microglia, APOE4-expressing microglia did not upregulate neurodegeneration-associated genes, including Lgals3, following IOP elevation. Genetic and pharmacologic targeting of Galectin-3 ameliorated RGC degeneration, and Galectin-3 expression was attenuated in human APOE4 glaucoma samples. These results demonstrate that impaired activation of APOE4 microglia is protective in glaucoma and that the APOE-Galectin-3 signaling can be targeted to treat this blinding disease.
Keywords: APOE4; Alzheimer disease; Galectin-3; Lgals3; glaucoma; microglia; neurodegeneration; neuroprotection; retina.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests O.B. and M.A.M. are co-inventors of a patent for the use of Galectin-3 inhibitors for the treatment of glaucoma. O.B. collaborates with Sanofi, GSK, and Regulus Therapeutics; receives research funding from Sanofi, GSK, and miRagen Therapeutics, and honoraria for lectures; and consults for GSK and Camp4. E.M.L. and A.D.P. receives research funding from Novartis Institutes for BioMedical Research and F. Hoffmann La Roche.
Figures



References
-
- Abreu CA, De Lima SV, Mendonca HR, Goulart CO, and Martinez AM (2017). Absence of galectin-3 promotes neuroprotection in retinal ganglion cells after optic nerve injury. Histol Histopathol 32, 253–262. - PubMed
-
- Almasieh M, and Levin LA (2017). Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu Rev Vis Sci 3, 91–120. - PubMed
-
- Alqawlaq S, Flanagan JG, and Sivak JM (2019). All roads lead to glaucoma: Induced retinal injury cascades contribute to a common neurodegenerative outcome. Exp Eye Res 183, 88–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM132668/GM/NIGMS NIH HHS/United States
- R21 NS101673/NS/NINDS NIH HHS/United States
- R41 EY025913/EY/NEI NIH HHS/United States
- K12 EY016335/EY/NEI NIH HHS/United States
- R01 AG054672/AG/NIA NIH HHS/United States
- R01 EY025259/EY/NEI NIH HHS/United States
- K08 EY030160/EY/NEI NIH HHS/United States
- P30 EY003790/EY/NEI NIH HHS/United States
- R21 NS104609/NS/NINDS NIH HHS/United States
- R01 AG051812/AG/NIA NIH HHS/United States
- R01 AG075509/AG/NIA NIH HHS/United States
- R44 EY025913/EY/NEI NIH HHS/United States
- R01 NS088137/NS/NINDS NIH HHS/United States
- R21 AG076982/AG/NIA NIH HHS/United States
- R01 EY027921/EY/NEI NIH HHS/United States
- K23 EY026988/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

