The spatiotemporal dynamics of microglia across the human lifespan
- PMID: 35977545
- PMCID: PMC9616795
- DOI: 10.1016/j.devcel.2022.07.015
The spatiotemporal dynamics of microglia across the human lifespan
Abstract
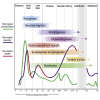
Microglia, the brain's resident macrophages, shape neural development and are key neuroimmune hubs in the pathological signatures of neurodevelopmental disorders. Despite the importance of microglia, their development has not been carefully examined in the human brain, and most of our knowledge derives from rodents. We aimed to address this gap in knowledge by establishing an extensive collection of 97 post-mortem tissues in order to enable quantitative, sex-matched, detailed analysis of microglia across the human lifespan. We identify the dynamics of these cells in the human telencephalon, describing waves in microglial density across gestation, infancy, and childhood, controlled by a balance of proliferation and apoptosis, which track key neurodevelopmental milestones. These profound changes in microglia are also observed in bulk RNA-seq and single-cell RNA-seq datasets. This study provides a detailed insight into the spatiotemporal dynamics of microglia across the human lifespan and serves as a foundation for elucidating how microglia contribute to shaping neurodevelopment in humans.
Keywords: RNA-seq; apoptosis; neurodevelopment; proliferation; single-cell RNA-seq.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Ameijeiras-Alonso J., Crujeiras R.M., Rodríguez-Casal A. Mode testing, critical bandwidth and excess mass. Test. 2019;28:900–919.
-
- Bayer S.A., Altman J. CRC Press; 2006. The human brain during the late first trimester. Atlas of Human Central Nervous System Development v 4.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

