Effectiveness of an intervention for reducing sitting time and improving health in office workers: three arm cluster randomised controlled trial
- PMID: 35977732
- PMCID: PMC9382450
- DOI: 10.1136/bmj-2021-069288
Effectiveness of an intervention for reducing sitting time and improving health in office workers: three arm cluster randomised controlled trial
Abstract
Objectives: To evaluate the effectiveness of an intervention, with and without a height adjustable desk, on daily sitting time, and to investigate the relative effectiveness of the two interventions, and the effectiveness of both interventions on physical behaviours and physical, biochemical, psychological, and work related health and performance outcomes.
Design: Cluster three arm randomised controlled trial with follow-up at three and 12 months.
Setting: Local government councils in Leicester, Liverpool, and Greater Manchester, UK.
Participants: 78 clusters including 756 desk based employees in defined offices, departments, or teams from two councils in Leicester, three in Greater Manchester, and one in Liverpool.
Interventions: Clusters were randomised to one of three conditions: the SMART Work and Life (SWAL) intervention, the SWAL intervention with a height adjustable desk (SWAL plus desk), or control (usual practice).
Main outcomes measures: The primary outcome measure was daily sitting time, assessed by accelerometry, at 12 month follow-up. Secondary outcomes were accelerometer assessed sitting, prolonged sitting, standing and stepping time, and physical activity calculated over any valid day, work hours, workdays, and non-workdays, self-reported lifestyle behaviours, musculoskeletal problems, cardiometabolic health markers, work related health and performance, fatigue, and psychological measures.
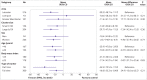
Results: Mean age of participants was 44.7 years, 72.4% (n=547) were women, and 74.9% (n=566) were white. Daily sitting time at 12 months was significantly lower in the intervention groups (SWAL -22.2 min/day, 95% confidence interval -38.8 to -5.7 min/day, P=0.003; SWAL plus desk -63.7 min/day, -80.1 to -47.4 min/day, P<0.001) compared with the control group. The SWAL plus desk intervention was found to be more effective than SWAL at changing sitting time (-41.7 min/day, -56.3 to -27.0 min/day, P<0.001). Favourable differences in sitting and prolonged sitting time at three and 12 month follow-ups for both intervention groups and for standing time for the SWAL plus desk group were observed during work hours and on workdays. Both intervention groups were associated with small improvements in stress, wellbeing, and vigour, and the SWAL plus desk group was associated with improvements in pain in the lower extremity, social norms for sitting and standing at work, and support.
Conclusions: Both SWAL and SWAL plus desk were associated with a reduction in sitting time, although the addition of a height adjustable desk was found to be threefold more effective.
Trial registration: ISRCTN Registry ISRCTN11618007.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Institute for Health and Care Research (NIHR) public health research programme, the Leicester Clinical Trials Unit, and the NIHR Leicester Biomedical Research Centre. MHG is a co-inventor of the activPAL3 physical activity monitor and a director of PAL Technologies. DWD reports grants from National Health and Medical Research Council (Australia), Diabetes Australia, and the National Heart Foundation of Australia, during the conduct of the study. GNH reports grants from the National Health and Medical Research Council (Australia) during the conduct of the study. MJD reports personal fees from Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp and Dohme, Boehringer Ingelheim, AstraZeneca, Janssen, Servier, Mitsubishi Tanabe Pharma, and Takeda Pharmaceuticals International, and grants from Novo Nordisk, Sanofi-Aventis, Lilly, Boehringer Ingelheim, and Janssen outside the submitted work.
Figures





References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
