Efficacy of Ubrogepant in the Acute Treatment of Migraine With Mild Pain vs Moderate or Severe Pain
- PMID: 35977836
- PMCID: PMC9620813
- DOI: 10.1212/WNL.0000000000201031
Efficacy of Ubrogepant in the Acute Treatment of Migraine With Mild Pain vs Moderate or Severe Pain
Abstract
Background and objectives: To examine the efficacy of ubrogepant in the treatment of migraine with mild vs moderate or severe pain.
Methods: This was a phase 3, open-label, dose-blinded, 52-week extension trial. Adults with migraine were randomized 1:1:1 (usual care, ubrogepant 50 mg, or ubrogepant 100 mg). Participants treated up to 8 migraine attacks of any pain intensity every 4 weeks. Efficacy outcomes (only collected for ubrogepant) included 2-hour pain freedom (2hPF), freedom from associated symptoms, and from disability. A generalized linear mixed model with binomial distribution and logit link function was used to assess the influence of baseline pain intensity on treatment outcomes in this post hoc analysis.
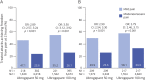
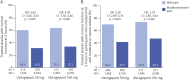
Results: Data for 19,291 attacks from 808 participants were included. 2hPF rates were higher for attacks treated when pain was mild vs moderate or severe: ubrogepant 50 mg (47.1% vs 23.6%; odds ratio [95% CI] 2.89 [2.57-3.24]) and ubrogepant 100 mg (55.2% vs 26.1%; 3.50 [3.12-3.92]; p < 0.0001 both doses). Rates of freedom from photophobia, phonophobia, and nausea 2 hours after treatment were also significantly higher following the treatment of mild vs moderate or severe pain (p < 0.001 all symptoms, both doses). At 2 hours, the proportion of attacks with normal function was more than double for both doses of ubrogepant (p < 0.001). The most common adverse event was upper respiratory tract infection (∼11% both doses). Serious adverse events were reported by 2% in ubrogepant 50 mg and 3% in ubrogepant 100 mg.
Discussion: Relative to treatment of attacks with moderate or severe pain, treatment with ubrogepant during mild pain resulted in significantly higher rates of freedom from pain, freedom from associated symptoms, and achieving normal function 2 hours after administration.
Trial registration information: ClinicalTrials.gov, NCT02873221.
Classification of evidence: This trial provides Class III evidence that treatment of migraine with ubrogepant when pain is mild vs moderate or severe increases the likelihood of achieving pain freedom, absence of symptoms, and normal function within 2 hours postdose.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures


Similar articles
-
Early Onset of Efficacy and Consistency of Response Across Multiple Migraine Attacks From the Randomized COMPASS Study: AVP-825 Breath Powered® Exhalation Delivery System (Sumatriptan Nasal Powder) vs Oral Sumatriptan.Headache. 2017 Jun;57(6):862-876. doi: 10.1111/head.13105. Epub 2017 May 11. Headache. 2017. PMID: 28497569 Clinical Trial.
-
Effect of Ubrogepant vs Placebo on Pain and the Most Bothersome Associated Symptom in the Acute Treatment of Migraine: The ACHIEVE II Randomized Clinical Trial.JAMA. 2019 Nov 19;322(19):1887-1898. doi: 10.1001/jama.2019.16711. JAMA. 2019. PMID: 31742631 Free PMC article. Clinical Trial.
-
Efficacy and safety of DFN-11 (sumatriptan injection, 3 mg) in adults with episodic migraine: an 8-week open-label extension study.J Headache Pain. 2018 Aug 15;19(1):70. doi: 10.1186/s10194-018-0882-y. J Headache Pain. 2018. PMID: 30112725 Free PMC article. Clinical Trial.
-
Safety and Efficacy of Ubrogepant for the Acute Treatment of Episodic Migraine: A Meta-Analysis of Randomized Clinical Trials.CNS Drugs. 2020 May;34(5):463-471. doi: 10.1007/s40263-020-00715-7. CNS Drugs. 2020. PMID: 32193827
-
Efficacy and safety of ubrogepant for migraine: a meta-analysis of randomized controlled studies.Int J Neurosci. 2024 Jun;134(2):124-130. doi: 10.1080/00207454.2022.2090351. Epub 2022 Oct 26. Int J Neurosci. 2024. PMID: 35999672
Cited by
-
Comparison of effectiveness and safety of lasmiditan and CGRP-antagonists for the acute treatment of migraine in adults: systematic review and network meta-analysis of randomised trials.J Headache Pain. 2024 Feb 5;25(1):16. doi: 10.1186/s10194-024-01723-4. J Headache Pain. 2024. PMID: 38311738 Free PMC article.
-
Saudi clinical practice guidelines for the treatment and prevention of migraine headache.Neurosciences (Riyadh). 2025 Apr;30(2):77-91. doi: 10.17712/nsj.2025.2.20240097. Neurosciences (Riyadh). 2025. PMID: 40199542 Free PMC article.
-
Effect of Ubrogepant on Patient-Reported Outcomes When Administered During the Migraine Prodrome: Results From the Randomized PRODROME Trial.Neurology. 2024 Sep 24;103(6):e209745. doi: 10.1212/WNL.0000000000209745. Epub 2024 Aug 28. Neurology. 2024. PMID: 39197113 Free PMC article. Clinical Trial.
-
Acute Treatment of Migraine: Expert Consensus Statements from the United Arab Emirates (UAE).Neurol Ther. 2024 Apr;13(2):257-281. doi: 10.1007/s40120-023-00576-4. Epub 2024 Jan 19. Neurol Ther. 2024. PMID: 38240944 Free PMC article.
-
A Comprehensive Review of the Mechanism, Efficacy, Safety, and Tolerability of Ubrogepant in the Treatment of Migraine.Cureus. 2023 Nov 2;15(11):e48160. doi: 10.7759/cureus.48160. eCollection 2023 Nov. Cureus. 2023. PMID: 38046695 Free PMC article. Review.
References
-
- Kelman L. Pain characteristics of the acute migraine attack. Headache. 2006;46(6):942-953. - PubMed
-
- Linde M, Mellberg A, Dahlöf C. The natural course of migraine attacks. A prospective analysis of untreated attacks compared with attacks treated with a triptan. Cephalalgia. 2006;26(6):712-721. - PubMed
-
- Headache Classification Committee of the International Headache Society IHS. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
