Disease Reactivation After Cessation of Disease-Modifying Therapy in Patients With Relapsing-Remitting Multiple Sclerosis
- PMID: 35977837
- PMCID: PMC9620810
- DOI: 10.1212/WNL.0000000000201029
Disease Reactivation After Cessation of Disease-Modifying Therapy in Patients With Relapsing-Remitting Multiple Sclerosis
Abstract
Background and objectives: To evaluate the rate of return of disease activity after cessation of multiple sclerosis (MS) disease-modifying therapy.
Methods: This was a retrospective cohort study from 2 large observational MS registries: MSBase and OFSEP. Patients with relapsing-remitting MS who had ceased a disease-modifying therapy and were followed up for the subsequent 12 months were included in the analysis. The primary study outcome was annualized relapse rate in the 12 months after disease-modifying therapy discontinuation stratified by patients who did, and did not, commence a subsequent therapy. The secondary endpoint was the predictors of first relapse and disability accumulation after treatment discontinuation.
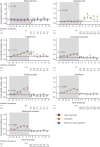
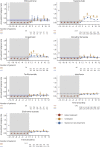
Results: A total of 14,213 patients, with 18,029 eligible treatment discontinuation epochs, were identified for 7 therapies. Annualized rates of relapse (ARRs) started to increase 2 months after natalizumab cessation (month 2-4 ARR 0.47, 95% CI 0.43-0.51). Commencement of a subsequent therapy within 2-4 months reduced the magnitude of disease reactivation (mean ARR difference: 0.15, 0.08-0.22). After discontinuation of fingolimod, rates of relapse increased overall (month 1-2 ARR: 0.80, 0.70-0.89) and stabilized faster in patients who started a new therapy within 1-2 months (mean ARR difference: 0.14, -0.01 to 0.29). The magnitude of disease reactivation for other therapies was low but reduced further by commencement of another treatment 1-10 months after treatment discontinuation. Predictors of relapse were a higher relapse rate in the year before cessation, female sex, younger age, and higher EDSS score. Commencement of a subsequent therapy reduced both the risk of relapse (HR 0.76, 95% CI 0.72-0.81) and disability accumulation (0.73, 0.65-0.80).
Discussion: The rate of disease reactivation after treatment cessation differs among MS treatments, with the peaks of relapse activity ranging from 1 to 10 months in untreated cohorts that discontinued different therapies. These results suggest that untreated intervals should be minimized after stopping antitrafficking therapies (natalizumab and fingolimod).
Classification of evidence: This study provides Class III that disease reactivation occurs within months of discontinuation of MS disease-modifying therapies. The risk of disease activity is reduced by commencement of a subsequent therapy.
© 2022 American Academy of Neurology.
Figures

References
-
- Kalincik T, Kubala Havrdova E, Horakova D, et al. Comparison of fingolimod, dimethyl fumarate and teriflunomide for multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(4):458-468. - PubMed
-
- Warrender-Sparkes M, Spelman T, Izquierdo G, et al. , MSBase Study Group. The effect of oral immunomodulatory therapy on treatment uptake and persistence in multiple sclerosis. Mult Scler. 2016;22(4):520-532. - PubMed
-
- Hartung HP, Gonsette R, Konig N, et al. , Mitoxantrone in Multiple Sclerosis Study Group MIMS. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018-2025. - PubMed
-
- Sorensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler. 2012;18(2):143-152. - PubMed
-
- Berkovich R. Clinical and MRI outcomes after stopping or switching disease-modifying therapy in stable MS patients: a case series report. Mult Scler Relat Disord. 2017;17:123-127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
