Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled trial
- PMID: 35977911
- PMCID: PMC9530565
- DOI: 10.1002/jcsm.13048
Efficacy of 12 weeks oral beta-alanine supplementation in patients with chronic obstructive pulmonary disease: a double-blind, randomized, placebo-controlled trial
Abstract
Background: Beta-alanine (BA) supplementation increases muscle carnosine, an abundant endogenous antioxidant and pH buffer in skeletal muscle. Carnosine loading promotes exercise capacity in healthy older adults. As patients with chronic obstructive pulmonary disease (COPD) suffer from elevated exercise-induced muscle oxidative/carbonyl stress and acidosis, and from reduced muscle carnosine stores, it was investigated whether BA supplementation augments muscle carnosine and induces beneficial changes in exercise capacity, quadriceps function, and muscle oxidative/carbonyl stress in patients with COPD.
Methods: In this double-blind, randomized, placebo (PL)-controlled trial (clinicaltrials.gov identifier: NCT02770417), 40 patients (75% male) with COPD (mean ± standard deviation: age 65 ± 6 years; FEV1 % predicted 55 ± 14%) were assigned to 12 weeks oral BA or PL supplementation (3.2 g/day). The primary outcome, i.e. muscle carnosine, was quantified from m. vastus lateralis biopsies obtained before and after intervention. Co-primary outcomes, i.e. incremental and constant work rate cycle capacity, were also assessed. Linear mixed model analyses were performed. Compliance with and side effects of supplement intake and secondary outcomes (quadriceps strength and endurance, and muscle oxidative/carbonyl stress) were also assessed.
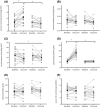
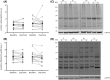
Results: Beta-alanine supplementation increased muscle carnosine in comparison with PL in patients with COPD (mean difference [95% confidence interval]; +2.82 [1.49-4.14] mmol/kg wet weight; P < 0.001). Maximal incremental cycling capacity (VO2 peak: +0.5 [-0.7 to 1.7] mL/kg/min; P = 0.384, Wpeak: +5 [-1 to 11] W; P = 0.103) and time to exhaustion on the constant work rate cycle test (+28 [-179 to 236] s; P = 0.782) did not change significantly. Compliance with supplement intake was similar in BA (median (quartile 1-quartile 3); 100 (98-100)%) and PL (98 (96-100)%) (P = 0.294) groups, and patients did not report side effects possibly related to supplement intake. No change was observed in secondary outcomes.
Conclusions: Beta-alanine supplementation is efficacious in augmenting muscle carnosine (+54% from mean baseline value) without side effects in patients with COPD in comparison with PL. However, accompanied beneficial changes in exercise capacity, quadriceps function, and muscle oxidative/carbonyl stress were not observed.
Keywords: Carnosine; Chronic obstructive pulmonary disease; Oxidative/carbonyl stress; Physical capacity.
© 2022 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
Jana De Brandt, Wim Derave, Frank Vandenabeele, Pascal Pomiès, Laura Blancquaert, Charly Keytsman, Marina S. Barusso‐Grüninger, Fabiano F. de Lima, Martijn A. Spruit, and Chris Burtin declare that they have no conflict of interest. Maurice Hayot has received research grants from Bastide Medical, which are not related to the current project; personal fees from AstraZeneca for participation to scientific lectures; financial support for congress participation from SOS Oxygène, Eole Santé, Boehringer Ingelheim, GlaxoSmithKline, and AstraZeneca; and hospitalities during local scientific meetings from ALK‐Abelló, Actelion Pharmaceuticals France, Vifor Fresenius Medical Care Renal Pharma, Sanofi Aventis France, Novartis Pharma, LVL Medical Sud, Chiesi, and SOS Oxygene Mediterranee.
Figures

References
-
- Maltais F, Simard AA, Simard C, Jobin J, Desgagnes P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 1996;153:288–293. - PubMed
-
- Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev 2013;93:1803–1845. - PubMed
-
- C. Harris R, Dunnett M, Greenhaff PL. Carnosine and taurine contents in individual fibres of human vastus lateralis muscle. J Sports Sci 1998;16:639–643.
-
- De Brandt J, Burtin C, Pomiès P, Vandenabeele F, Verboven K, Aumann J, et al. Carnosine, oxidative and carbonyl stress, antioxidants and muscle fiber characteristics of quadriceps muscle of patients with COPD. J Appl Physiol 1985;2021. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

