m6A RNA methylation-mediated NDUFA4 promotes cell proliferation and metabolism in gastric cancer
- PMID: 35977935
- PMCID: PMC9385701
- DOI: 10.1038/s41419-022-05132-w
m6A RNA methylation-mediated NDUFA4 promotes cell proliferation and metabolism in gastric cancer
Abstract
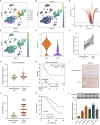
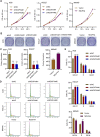
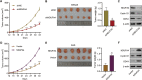
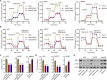
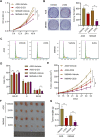
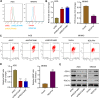
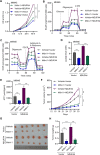
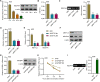
Gastric cancer (GC) is a malignancy with poor prognosis. NDUFA4 is reported to correlate with the progression of GC. However, its underlying mechanism in GC is unknown. Our study was to reveal the pathogenic mechanism of NDUFA4 in GC. NDUFA4 expression was explored in single-cell and bulk RNA-seq data as well as GC tissue microarray. Mitochondrial respiration and glycolysis were estimated by oxygen consumption rate and extracellular acidification rate, respectively. The interaction between NDUFA4 and METTL3 was validated by RNA immunoprecipitation. Flow cytometry was used to estimate cell cycle, apoptosis and mitochondrial activities. NDUFA4 was highly expressed in GC and its high expression indicated a poor prognosis. The knockdown of NDUFA4 could reduce cell proliferation and inhibit tumor growth. Meanwhile, NDUFA4 could promote glycolytic and oxidative metabolism in GC cells, whereas the inhibition of glycolysis suppressed the proliferation and tumor growth of GC. Besides, NDUFA4 inhibited ROS level and promoted MMP level in GC cells, whereas the inhibition of mitochondrial fission could reverse NDUFA4-induced glycolytic and oxidative metabolism and tumor growth of GC. Additionally, METTL3 could increase the m6A level of NDUFA4 mRNA via the m6A reader IGF2BP1 to promote NDUFA4 expression in GC cells. Our study revealed that NDUFA4 was increased by m6A methylation and could promote GC development via enhancing cell glycolysis and mitochondrial fission. NDUFA4 was a potential target for GC treatment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet (Lond, Engl) 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

