A lymphatic-absorbed multi-targeted kinase inhibitor for myelofibrosis therapy
- PMID: 35977945
- PMCID: PMC9386018
- DOI: 10.1038/s41467-022-32486-8
A lymphatic-absorbed multi-targeted kinase inhibitor for myelofibrosis therapy
Abstract
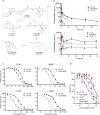
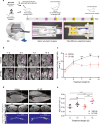
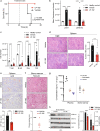
Activation of compensatory signaling nodes in cancer often requires combination therapies that are frequently plagued by dose-limiting toxicities. Intestinal lymphatic drug absorption is seldom explored, although reduced toxicity and sustained drug levels would be anticipated to improve systemic bioavailability. A potent orally bioavailable multi-functional kinase inhibitor (LP-182) is described with intrinsic lymphatic partitioning for the combined targeting of phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) signaling pathways without observable toxicity. We demonstrate selectivity and therapeutic efficacy through reduction of downstream kinase activation, amelioration of disease phenotypes, and improved survival in animal models of myelofibrosis. Our further characterization of synthetic and physiochemical properties for small molecule lymphatic uptake will support continued advancements in lymphatropic therapy for altering disease trajectories of a myriad of human disease indications.
© 2022. The Author(s).
Conflict of interest statement
B.D.R. and M.V.D. are inventors on patents describing the underlying compounds owned by the University of Michigan and may receive royalty payments from the University. Compound patents have been licensed to
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

