Wearable adjunct ozone and antibiotic therapy system for treatment of Gram-negative dermal bacterial infection
- PMID: 35977975
- PMCID: PMC9385669
- DOI: 10.1038/s41598-022-17495-3
Wearable adjunct ozone and antibiotic therapy system for treatment of Gram-negative dermal bacterial infection
Abstract
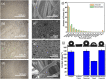
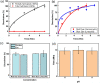
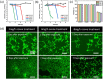
The problematic combination of a rising prevalence of skin and soft tissue infections and the growing rate of life-threatening antibiotic resistant infections presents an urgent, unmet need for the healthcare industry. These evolutionary resistances originate from mutations in the bacterial cell walls which prevent effective diffusion of antibiotics. Gram-negative bacteria are of special consideration due to the natural resistance to many common antibiotics due to the unique bilayer structure of the cell wall. The system developed here provides one solution to this problem through a wearable therapy that delivers and utilizes gaseous ozone as an adjunct therapy with topical antibiotics through a novel dressing with drug-eluting nanofibers (NFs). This technology drastically increases the sensitivity of Gram-negative bacteria to common antibiotics by using oxidative ozone to bypass resistances created by the bacterial cell wall. To enable simple and effective application of adjunct therapy, ozone delivery and topical antibiotics have been integrated into a single application patch. The drug delivery NFs are generated via electrospinning in a fast-dissolve PVA mat without inducing decreasing gas permeability of the dressing. A systematic study found ozone generation at 4 mg/h provided optimal ozone levels for high antimicrobial performance with minimal cytotoxicity. This ozone treatment was used with adjunct therapy delivered by the system in vitro. Results showed complete eradication of Gram-negative bacteria with ozone and antibiotics typically used only for Gram-positive bacteria, which showed the strength of ozone as an enabling adjunct treatment option to sensitize bacteria strains to otherwise ineffective antibiotics. Furthermore, the treatment is shown through biocompatibility testing to exhibit no cytotoxic effect on human fibroblast cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Niska, R., Bhuiya, F. & Xu, J. National hospital ambulatory medical care survey: 2016 Emergency department summary. National Health Statistics Reports (2016). - PubMed
-
- Skin Soft Tissue Infections (SSTI) Care and Process Models in Adults. (2018).
-
- Report, N. D. S. National Diabetes Statistics Report, 2020. Natl. Diabetes Stat. Rep. Vol. 2. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-stat... (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

