Low pH structure of heliorhodopsin reveals chloride binding site and intramolecular signaling pathway
- PMID: 35977989
- PMCID: PMC9385722
- DOI: 10.1038/s41598-022-17716-9
Low pH structure of heliorhodopsin reveals chloride binding site and intramolecular signaling pathway
Abstract
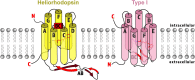
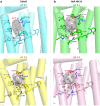
Within the microbial rhodopsin family, heliorhodopsins (HeRs) form a phylogenetically distinct group of light-harvesting retinal proteins with largely unknown functions. We have determined the 1.97 Å resolution X-ray crystal structure of Thermoplasmatales archaeon SG8-52-1 heliorhodopsin (TaHeR) in the presence of NaCl under acidic conditions (pH 4.5), which complements the known 2.4 Å TaHeR structure acquired at pH 8.0. The low pH structure revealed that the hydrophilic Schiff base cavity (SBC) accommodates a chloride anion to stabilize the protonated retinal Schiff base when its primary counterion (Glu-108) is neutralized. Comparison of the two structures at different pH revealed conformational changes connecting the SBC and the extracellular loop linking helices A-B. We corroborated this intramolecular signaling transduction pathway with computational studies, which revealed allosteric network changes propagating from the perturbed SBC to the intracellular and extracellular space, suggesting TaHeR may function as a sensory rhodopsin. This intramolecular signaling mechanism may be conserved among HeRs, as similar changes were observed for HeR 48C12 between its pH 8.8 and pH 4.3 structures. We additionally performed DEER experiments, which suggests that TaHeR forms possible dimer-of-dimer associations which may be integral to its putative functionality as a light sensor in binding a transducer protein.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
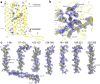


Similar articles
-
Internal Proton Transfer in the Activation of Heliorhodopsin.J Mol Biol. 2024 Mar 1;436(5):168273. doi: 10.1016/j.jmb.2023.168273. Epub 2023 Sep 12. J Mol Biol. 2024. PMID: 37709010
-
High-resolution structural insights into the heliorhodopsin family.Proc Natl Acad Sci U S A. 2020 Feb 25;117(8):4131-4141. doi: 10.1073/pnas.1915888117. Epub 2020 Feb 7. Proc Natl Acad Sci U S A. 2020. PMID: 32034096 Free PMC article.
-
Specific zinc binding to heliorhodopsin.Phys Chem Chem Phys. 2023 Jan 27;25(4):3535-3543. doi: 10.1039/d2cp04718g. Phys Chem Chem Phys. 2023. PMID: 36637167
-
Microbial Rhodopsins: The Last Two Decades.Annu Rev Microbiol. 2021 Oct 8;75:427-447. doi: 10.1146/annurev-micro-031721-020452. Epub 2021 Aug 3. Annu Rev Microbiol. 2021. PMID: 34343014 Review.
-
Coupling of protonation switches during rhodopsin activation.Photochem Photobiol. 2007 Mar-Apr;83(2):286-92. doi: 10.1562/2006-06-19-IR-937. Photochem Photobiol. 2007. PMID: 17576345 Review.
Cited by
-
pH-Dependent Binding and Releasing Mechanism of Acetate in the Inner Water Cavity of Heliorhodopsin.Biochemistry. 2023 Aug 15;62(16):2363-2370. doi: 10.1021/acs.biochem.3c00193. Epub 2023 Jul 20. Biochemistry. 2023. PMID: 37471424 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

