Molecular Quantity Variations in Human-Mandibular-Bone Osteoid
- PMID: 35978052
- PMCID: PMC9613710
- DOI: 10.1007/s00223-022-01017-4
Molecular Quantity Variations in Human-Mandibular-Bone Osteoid
Abstract
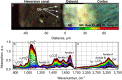
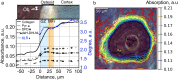
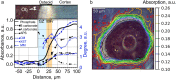
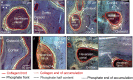
Osteoid is a layer of new-formed bone that is deposited on the bone border during the process of new bone formation. This deposition process is crucial for bone tissue, and flaws in it can lead to bone diseases. Certain bone diseases, i.e. medication related osteonecrosis, are overexpressed in mandibular bone. Because mandibular bone presents different properties than other bone types, the data concerning osteoid formation in other bones are inapplicable for human-mandibular bone. Previously, the molecular distribution of other bone types has been presented using Fourier-transform infrared (FTIR) spectroscopy. However, the spatial distribution of molecular components of healthy-human-mandibular-bone osteoid in relation to histologic landmarks has not been previously presented and needs to be studied in order to understand diseases that occur human-mandibular bone. This study presents for the first time the variation in molecular distribution inside healthy-human-mandibular-bone osteoid by juxtaposing FTIR data with its corresponding histologic image obtained by autofluorescence imaging of its same bone section. During new bone formation, bone-forming cells produce an osteoid constituted primarily of type I collagen. It was observed that in mandibular bone, the collagen type I increases from the osteoblast line with the distance from the osteoblasts, indicating progressive accumulation of collagen during osteoid formation. Only later inside the collagen matrix, the osteoid starts to mineralize. When the mineralization starts, the collagen accumulation diminishes whereas the collagen maturation still continues. This chemical-apposition process in healthy mandibular bone will be used in future as a reference to understand different pathologic conditions that occur in human-mandibular bone.
Keywords: Bone FTIR; Bone autofluorescence; Bone modeling and remodeling; Collagen; Matrix mineralization.
© 2022. The Author(s).
Conflict of interest statement
Anni Palander, Laure Fauch, Mikael J. Turunen, Hannah Dekker, Engelbert A. J. M. Schulten, Arto Koistinen, Nathalie Bravenboer, Arja Kullaa have no conflict of interest.
Figures

Similar articles
-
Effects of ethnicity and age or menopause on osteoblast function, bone mineralization, and osteoid accumulation in iliac bone.J Bone Miner Res. 1997 Nov;12(11):1864-73. doi: 10.1359/jbmr.1997.12.11.1864. J Bone Miner Res. 1997. PMID: 9383691
-
Fourier transform infrared imaging as a tool to chemically and spatially characterize matrix-mineral deposition in osteoblasts.Calcif Tissue Int. 2013 Jan;92(1):50-8. doi: 10.1007/s00223-012-9667-5. Epub 2012 Nov 10. Calcif Tissue Int. 2013. PMID: 23143076
-
The primary calcification in bones follows removal of decorin and fusion of collagen fibrils.J Bone Miner Res. 1999 Feb;14(2):273-80. doi: 10.1359/jbmr.1999.14.2.273. J Bone Miner Res. 1999. PMID: 9933482
-
The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological and kinetic approaches to bone turnover.Metabolism. 1976 Jul;25(7):809-44. doi: 10.1016/0026-0495(76)90151-7. Metabolism. 1976. PMID: 781470 Review.
-
Bone disease and aluminum: pathogenic considerations.Am J Kidney Dis. 1985 Nov;6(5):330-5. doi: 10.1016/s0272-6386(85)80089-5. Am J Kidney Dis. 1985. PMID: 3904425 Review.
References
-
- Adler HH. Infrared spectra of phosphate minerals: symmetry and substitutional effects in the pyromorphite series. Am Min. 1964;49(7–8):1002–1015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

