Microbiota mitochondria disorders as hubs for early age-related macular degeneration
- PMID: 35978068
- PMCID: PMC9385247
- DOI: 10.1007/s11357-022-00620-5
Microbiota mitochondria disorders as hubs for early age-related macular degeneration
Abstract
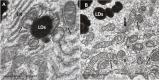
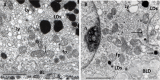
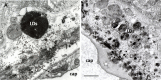
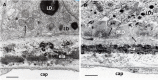
Age-related macular degeneration (AMD) is a progressive neurodegenerative disease affecting the central area (macula lutea) of the retina. Research on the pathogenic mechanism of AMD showed complex cellular contribution governed by such risk factors as aging, genetic predisposition, diet, and lifestyle. Recent studies suggested that microbiota is a transducer and a modifier of risk factors for neurodegenerative diseases, and mitochondria may be one of the intracellular targets of microbial signaling molecules. This review explores studies supporting a new concept on the contribution of microbiota-mitochondria disorders to AMD. We discuss metabolic, vascular, immune, and neuronal mechanism in AMD as well as key alterations of photoreceptor cells, retinal pigment epithelium (RPE), Bruch's membrane, choriocapillaris endothelial, immune, and neuronal cells. Special attention was paid to alterations of mitochondria contact sites (MCSs), an organelle network of mitochondria, endoplasmic reticulum, lipid droplets (LDs), and peroxisomes being documented based on our own electron microscopic findings from surgically removed human eyes. Morphometry of Bruch's membrane lipids and proteoglycans has also been performed in early AMD and aged controls. Microbial metabolites (short-chain fatty acids, polyphenols, and secondary bile acids) and microbial compounds (lipopolysaccharide, peptidoglycan, and bacterial DNA)-now called postbiotics-in addition to local effects on resident microbiota and mucous membrane, regulate systemic metabolic, vascular, immune, and neuronal mechanisms in normal conditions and in various common diseases. We also discuss their antioxidant, anti-inflammatory, and metabolic effects as well as experimental and clinical observations on regulating the main processes of photoreceptor renewal, mitophagy, and autophagy in early AMD. These findings support an emerging concept that microbiota-mitochondria disorders may be a crucial pathogenic mechanism of early AMD; and similarly, to other age-related neurodegenerative diseases, new treatment approaches should be targeted at these disorders.
Keywords: Age-related macular degeneration; Bruch’s membrane; Choriocapillaris; Electron microscopy; Ferroptosis; Innate immunity; Lipid droplets; Microbiota; Microglia; Mitochondria; Mitochondria contact sites; Morphometry; Photoreceptor; Retinal pigment epithelium.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, et al. An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiological Study Group. Surv Ophthalmol. 1995;39:367–374. doi: 10.1016/s0039-6257(05)80092-x. - DOI - PubMed
-
- Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–636. doi: 10.1016/j.ophtha.2019.11.004. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

