Formulation Excipients and Their Role in Insulin Stability and Association State in Formulation
- PMID: 35978148
- PMCID: PMC9633423
- DOI: 10.1007/s11095-022-03367-y
Formulation Excipients and Their Role in Insulin Stability and Association State in Formulation
Abstract
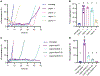
While excipients are often overlooked as the "inactive" ingredients in pharmaceutical formulations, they often play a critical role in protein stability and absorption kinetics. Recent work has identified an ultrafast absorbing insulin formulation that is the result of excipient modifications. Specifically, the insulin monomer can be isolated by replacing zinc and the phenolic preservative metacresol with phenoxyethanol as an antimicrobial agent and an amphiphilic acrylamide copolymer excipient for stability. A greater understanding is needed of the interplay between excipients, insulin association state, and stability in order to optimize this formulation. Here, we formulated insulin with different preservatives and stabilizing excipient concentrations using both insulin lispro and regular human insulin and assessed the insulin association states using analytical ultracentrifugation as well as formulation stability. We determined that phenoxyethanol is required to eliminate hexamers and promote a high monomer content even in a zinc-free lispro formulation. There is also a concentration dependent relationship between the concentration of polyacrylamide-based copolymer excipient and insulin stability, where a concentration greater than 0.1 g/mL copolymer is required for a mostly monomeric zinc-free lispro formulation to achieve stability exceeding that of Humalog in a stressed aging assay. Further, we determined that under the formulation conditions tested zinc-free regular human insulin remains primarily hexameric and is not at this time a promising candidate for rapid-acting formulations.
Keywords: aggregation; diabetes; excipient; insulin; polymer.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures
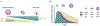



Similar articles
-
An ultrafast insulin formulation enabled by high-throughput screening of engineered polymeric excipients.Sci Transl Med. 2020 Jul 1;12(550):eaba6676. doi: 10.1126/scitranslmed.aba6676. Sci Transl Med. 2020. PMID: 32611683 Free PMC article.
-
Stable Monomeric Insulin Formulations Enabled by Supramolecular PEGylation of Insulin Analogues.Adv Ther (Weinh). 2020 Jan;3(1):1900094. doi: 10.1002/adtp.201900094. Epub 2019 Dec 17. Adv Ther (Weinh). 2020. PMID: 32190729 Free PMC article.
-
Photoacoustic imaging reveals mechanisms of rapid-acting insulin formulations dynamics at the injection site.Mol Metab. 2022 Aug;62:101522. doi: 10.1016/j.molmet.2022.101522. Epub 2022 Jun 4. Mol Metab. 2022. PMID: 35671972 Free PMC article.
-
A review of a family of ultra-rapid-acting insulins: formulation development.J Diabetes Sci Technol. 2012 Jul 1;6(4):786-96. doi: 10.1177/193229681200600408. J Diabetes Sci Technol. 2012. PMID: 22920803 Free PMC article. Review.
-
The Stabilizing Excipients in Dry State Therapeutic Phage Formulations.AAPS PharmSciTech. 2020 May 15;21(4):133. doi: 10.1208/s12249-020-01673-5. AAPS PharmSciTech. 2020. PMID: 32415395 Review.
Cited by
-
A temperature-responsive dual-hormone foam nanoengine improves rectal absorptivity of insulin-pramlintide for diabetes treatment.Sci Adv. 2024 Aug 30;10(35):eadn8695. doi: 10.1126/sciadv.adn8695. Epub 2024 Aug 28. Sci Adv. 2024. PMID: 39196940 Free PMC article.
-
Insulin therapy in type 2 diabetes: Insights into clinical efficacy, patient-reported outcomes, and adherence challenges.World J Diabetes. 2024 May 15;15(5):828-852. doi: 10.4239/wjd.v15.i5.828. World J Diabetes. 2024. PMID: 38766443 Free PMC article. Review.
-
Comparative physicochemical and structural characterisation studies establish high biosimilarity between BGL-ASP and reference insulin aspart.Sci Rep. 2024 Feb 20;14(1):4224. doi: 10.1038/s41598-024-54819-x. Sci Rep. 2024. PMID: 38378730 Free PMC article.
References
-
- International Diabetes Federation IDF Diabetes Atlas, 9th edn.. 2019. https://www.diabetesatlas.org.
-
- Tillil H, Shapiro ET, Miller MA, Karrison T, Frank BH, Galloway JA, Rubenstein AH, Polonsky KS. Dose-dependent effects of oral and intravenous glucose on insulin secretion and clearance in normal humans. Am J Physiol. 1988;254:E349–57. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

