MYB orchestrates T cell exhaustion and response to checkpoint inhibition
- PMID: 35978192
- PMCID: PMC9452299
- DOI: 10.1038/s41586-022-05105-1
MYB orchestrates T cell exhaustion and response to checkpoint inhibition
Abstract
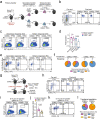
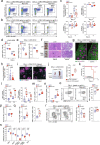
CD8+ T cells that respond to chronic viral infections or cancer are characterized by the expression of inhibitory receptors such as programmed cell death protein 1 (PD-1) and by the impaired production of cytokines. This state of restrained functionality-which is referred to as T cell exhaustion1,2-is maintained by precursors of exhausted T (TPEX) cells that express the transcription factor T cell factor 1 (TCF1), self-renew and give rise to TCF1- exhausted effector T cells3-6. Here we show that the long-term proliferative potential, multipotency and repopulation capacity of exhausted T cells during chronic infection are selectively preserved in a small population of transcriptionally distinct CD62L+ TPEX cells. The transcription factor MYB is not only essential for the development of CD62L+ TPEX cells and maintenance of the antiviral CD8+ T cell response, but also induces functional exhaustion and thereby prevents lethal immunopathology. Furthermore, the proliferative burst in response to PD-1 checkpoint inhibition originates exclusively from CD62L+ TPEX cells and depends on MYB. Our findings identify CD62L+ TPEX cells as a stem-like population that is central to the maintenance of long-term antiviral immunity and responsiveness to immunotherapy. Moreover, they show that MYB is a transcriptional orchestrator of two fundamental aspects of exhausted T cell responses: the downregulation of effector function and the long-term preservation of self-renewal capacity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures














Comment in
-
Defining the 'exhausted' T cells that respond to PD1 checkpoint blockade.Nat Rev Immunol. 2022 Oct;22(10):593. doi: 10.1038/s41577-022-00778-1. Nat Rev Immunol. 2022. PMID: 36064779 No abstract available.
-
c-Myb redefines the hierarchy of stem-like T cells.Nat Immunol. 2022 Oct;23(10):1405-1407. doi: 10.1038/s41590-022-01319-7. Nat Immunol. 2022. PMID: 36192534 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

