Fungal diversity in the gut microbiome of young South African children
- PMID: 35978282
- PMCID: PMC9387017
- DOI: 10.1186/s12866-022-02615-w
Fungal diversity in the gut microbiome of young South African children
Abstract
Background: The fungal microbiome, or mycobiome, is a poorly described component of the gut ecosystem and little is known about its structure and development in children. In South Africa, there have been no culture-independent evaluations of the child gut mycobiota. This study aimed to characterise the gut mycobiota and explore the relationships between fungi and bacteria in the gut microbiome of children from Cape Town communities.
Methods: Stool samples were collected from children enrolled in the TB-CHAMP clinical trial. Internal transcribed spacer 1 (ITS1) gene sequencing was performed on a total of 115 stool samples using the Illumina MiSeq platform. Differences in fungal diversity and composition in relation to demographic, clinical, and environmental factors were investigated, and correlations between fungi and previously described bacterial populations in the same samples were described.
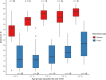
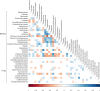
Results: Taxa from the genera Candida and Saccharomyces were detected in all participants. Differential abundance analysis showed that Candida spp. were significantly more abundant in children younger than 2 years compared to older children. The gut mycobiota was less diverse than the bacterial microbiota of the same participants, consistent with the findings of other human microbiome studies. The variation in richness and evenness of fungi was substantial, even between individuals of the same age. There was significant association between vitamin A supplementation and higher fungal alpha diversity (p = 0.047), and girls were shown to have lower fungal alpha diversity (p = 0.003). Co-occurrence between several bacterial taxa and Candida albicans was observed.
Conclusions: The dominant fungal taxa in our study population were similar to those reported in other paediatric studies; however, it remains difficult to identify the true core gut mycobiota due to the challenges set by the low abundance of gut fungi and the lack of true gut colonising species. The connection between the microbiota, vitamin A supplementation, and growth and immunity warrants exploration, especially in populations at risk for micronutrient deficiencies. While we were able to provide insight into the gut mycobiota of young South African children, further functional studies are necessary to explain the role of the mycobiota and the correlations between bacteria and fungi in human health.
Keywords: Children; Gut fungi; ITS; Microbiome; Mycobiota.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

