Impact of water deficiency on leaf cuticle lipids and gene expression networks in cotton (Gossypium hirsutum L.)
- PMID: 35978290
- PMCID: PMC9382817
- DOI: 10.1186/s12870-022-03788-2
Impact of water deficiency on leaf cuticle lipids and gene expression networks in cotton (Gossypium hirsutum L.)
Abstract
Background: Water deficit (WD) has serious effect on the productivity of crops. Formation of cuticular layer with increased content of wax and cutin on leaf surfaces is closely related to drought tolerance. Identification of drought tolerance associated wax components and cutin monomers and the genes responsible for their biosynthesis is essential for understanding the physiological and genetic mechanisms underlying drought tolerance and improving crop drought resistance.
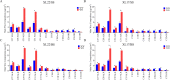
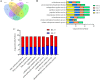
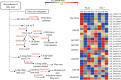
Result: In this study, we conducted comparative phenotypic and transcriptomic analyses of two Gossypium hirsutum varieties that are tolerant (XL22) or sensitive (XL17) to drought stress. XL17 consumed more water than XL22, particularly under the WD conditions. WD significantly induced accumulation of most major wax components (C29 and C31 alkanes) and cutin monomers (palmitic acid and stearic acid) in leaves of both XL22 and XL17, although accumulation of the major cutin monomers, i.e., polyunsaturated linolenic acid (C18:3n-3) and linoleic acid (C18:2n-6), were significantly repressed by WD in both XL22 and XL17. According to the results of transcriptome analysis, although many genes and their related pathways were commonly induced or repressed by WD in both XL22 and XL17, WD-induced differentially expressed genes specific to XL22 or XL17 were also evident. Among the genes that were commonly induced by WD were the GhCER1 genes involved in biosynthesis of alkanes, consistent with the observation of enhanced accumulation of alkanes in cotton leaves under the WD conditions. Interestingly, under the WD conditions, several GhCYP86 genes, which encode enzymes catalyzing the omega-hydroxylation of fatty acids and were identified to be the hub genes of one of the co-expression gene modules, showed a different expression pattern between XL22 and XL17 that was in agreement with the WD-induced changes of the content of hydroxyacids or fatty alcohols in these two varieties.
Conclusion: The results contribute to our comprehending the physiological and genetic mechanisms underlying drought tolerance and provide possible solutions for the difference of drought resistance of different cotton varieties.
Keywords: Co-expression gene network; Cutin monomers; Differentially expressed genes; Gossypium hirsutum; Transcriptomic analysis; Wax components.
© 2022. The Author(s).
Conflict of interest statement
Not applicable.
Figures






References
-
- Wu H, Shi S, Lu X, Li T, Wang J, Liu T, Zhang Q, Sun W, Li C, Wang Z, Chen Y, Quan L. Expression analysis and functional characterization of CER1 family genes involved in very-long-chain alkanes biosynthesis in Brachypodium distachyon. Front Plant Sci. 2019;10:1389. doi: 10.3389/fpls.2019.01389. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

