Testing P2Y12 platelet inhibitors generics beyond bioequivalence: a parallel single-blinded randomized trial
- PMID: 35978315
- PMCID: PMC9382000
- DOI: 10.1186/s12959-022-00405-y
Testing P2Y12 platelet inhibitors generics beyond bioequivalence: a parallel single-blinded randomized trial
Abstract
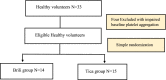
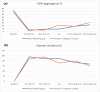
Cardiovascular diseases are the leading cause of death worldwide. Ticagrelor is an oral antiplatelet drug used in acute coronary syndrome. Although generic drugs are approved for their bioequivalence to the original product, they are not necessarily to be therapeutically equivalent. This study was conducted to prove the efficacy and safety of ticagrelor generically named Ticaloguard® compared to its brand Brilique® in healthy volunteers. A loading dose of 180 mg ticagrelor named Brilique® or Ticaloguard® followed by a 90 mg twice daily regimen as maintenance dose was given to 14 and 15 volunteers in Tica and Brili groups, respectively. The platelet aggregation on the ADP agonist was assessed at baseline and repeated 1 h and 3 h after the loading dose, on day 4 (after reaching steady-state), 12 and 24 h after discontinuation of the antiplatelet drug. Adverse effects from trial medications were noted by direct questions. It was shown that generic Ticaloguard® provides a similar therapeutic effect and safety as its branded Brilique® (p > 0.05). This will permit safe and trusted use of the generic Ticaloguard® when treating it in the same manner as Brilique®. Testing generic drug effects rather than simple bioequivalency, especially for drugs that are used in critical life-threatening situations, is crucial. We advocate applying this form of a clinical trial to test surrogate clinical efficacy for generics used in critical indications before having real-world data whenever possible.
Keywords: Acute coronary syndrome; Ticagrelor. Generics.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: A randomized, crossover clinical trial.PLoS Med. 2017 Nov 14;14(11):e1002428. doi: 10.1371/journal.pmed.1002428. eCollection 2017 Nov. PLoS Med. 2017. PMID: 29135993 Free PMC article. Clinical Trial.
-
Pharmacodynamic Comparison of Prasugrel Versus Ticagrelor in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)-4 Study.Circulation. 2016 Sep 13;134(11):780-92. doi: 10.1161/CIRCULATIONAHA.116.023402. Epub 2016 Aug 24. Circulation. 2016. PMID: 27559041 Clinical Trial.
-
Ticagrelor: an investigational oral antiplatelet treatment for reduction of major adverse cardiac events in patients with acute coronary syndrome.Vasc Health Risk Manag. 2010 Oct 21;6:963-77. doi: 10.2147/VHRM.S13263. Vasc Health Risk Manag. 2010. PMID: 21057581 Free PMC article. Review.
-
Effects of Clopidogrel, Prasugrel and Ticagrelor on Microvascular Function and Platelet Reactivity in Patients With Acute Coronary Syndrome Undergoing Coronary Artery Stenting. A Randomized, Blinded, Parallel Group Trial.Front Cardiovasc Med. 2021 Dec 13;8:780605. doi: 10.3389/fcvm.2021.780605. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34966798 Free PMC article.
-
Current Status of Antiplatelet Therapy in Acute Coronary Syndrome.Cardiovasc Hematol Agents Med Chem. 2015;13(1):40-9. doi: 10.2174/187152571301150730114514. Cardiovasc Hematol Agents Med Chem. 2015. PMID: 26245659 Review.
Cited by
-
Can Generic Medications Be a Safe and Effective Alternative to Brand-Name Drugs for Cardiovascular Disease Treatment? A Systematic Review and Meta-Analysis.Rev Cardiovasc Med. 2025 Mar 7;26(3):26116. doi: 10.31083/RCM26116. eCollection 2025 Mar. Rev Cardiovasc Med. 2025. PMID: 40160563 Free PMC article.
References
-
- El-Adawy AH, Elkhawaga G, Elraheem SA, Mahfouz E. Impact of socioeconomic status on in-hospital outcome of patients with acute coronary syndrome in Egypt. Clin Med Res. 2017;6(6):164–172. doi: 10.11648/j.cmr.20170606.11. - DOI
-
- World Health Organization. Cardiovascular diseases (CVDs). Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases...) [cited 2021 Dec 24].
LinkOut - more resources
Full Text Sources