Triple MAPK inhibition salvaged a relapsed post-BCMA CAR-T cell therapy multiple myeloma patient with a BRAF V600E subclonal mutation
- PMID: 35978321
- PMCID: PMC9382834
- DOI: 10.1186/s13045-022-01330-3
Triple MAPK inhibition salvaged a relapsed post-BCMA CAR-T cell therapy multiple myeloma patient with a BRAF V600E subclonal mutation
Erratum in
-
Correction: Triple MAPK inhibition salvaged a relapsed post-BCMA CAR-T cell therapy multiple myeloma patient with a BRAF V600E subclonal mutation.J Hematol Oncol. 2023 May 8;16(1):52. doi: 10.1186/s13045-023-01449-x. J Hematol Oncol. 2023. PMID: 37158935 Free PMC article. No abstract available.
Abstract
Background: Multiple Myeloma (MM) is a progressive plasma cell neoplasm characterized by heterogeneous clonal expansion. Despite promising response rates achieved with anti-BCMA CAR-T cell therapy, patients may still relapse and there are currently no clear therapeutic options in post-CAR-T settings. In this report, we present a case of a post-BCMA CAR-T relapsed/refractory (RR) MM patient with skin extramedullary disease (EMD) in which a novel MAPK inhibition combinatorial strategy was implemented based on next-generation sequencing and in vitro experiments.
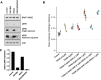
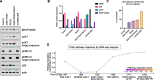
Case presentation: A 61-year-old male with penta-refractory MM penta- (IgA lambda), ISS stage 3 with hyperdiploidy, gain of 1q21 and del13 was treated with anti-BCMA CAR-T cell therapy, achieving a best response of VGPR. He progressed after 6 months and was salvaged for a short period with autologous stem cell transplantation. Eventually, he progressed with extramedullary disease manifested as subcutaneous nodules. Based on whole-exome sequencing, we identified a BRAF (V600E) dominant subclone in both bone marrow and cutaneous plasmacytoma. Following in vitro experiments, and according to our previous studies, we implemented a triple MAPK inhibition strategy under which the patient achieved a very good partial response for 110 days, which allowed to bridge him to subsequent clinical trials and eventually achieve a stringent complete response (sCR).
Conclusion: Here, we show the applicability, effectiveness, and tolerability the triple MAPK inhibition strategy in the context of post-BCMA CAR-T failure in specific subset of patients. The triple therapy could bridge our hospice bound RRMM patient with BRAF (V600E) to further therapeutic options where sCR was achieved. We will further evaluate triple MAPK inhibition in patients with BRAF V600E in a precision medicine clinical trial launching soon.
Keywords: BCMA CAR-T relapse; BRAF (V600E); Clonality; MAPK inhibition; Multiple myeloma; RNA-seq; Whole-exome sequencing.
© 2022. The Author(s).
Conflict of interest statement
P.I. Poulikakos reports research grants to the Institution by Black Diamond Therapeutics and Verastem Oncology. Sundar Jagannath reports consulting fees for Bristol Myers Squibb (Celgene), Janssen, Karyopharm Therapeutics, Merck, Sanofi, and Takeda Pharmaceuticals. Samir Parekh reports consulting fees from Foundation Medicine and research funding from Bristol Myers Squibb (Celgene), Karyopharm, and Amgen.
Figures


References
-
- Corcoran RB, et al. EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–235. doi: 10.1158/2159-8290.CD-11-0341. - DOI - PMC - PubMed
-
- Adamopoulos C, et al. Exploiting allosteric properties of RAF and MEK inhibitors to target therapy-resistant tumors driven by oncogenic BRAF signaling. Cancer Discov. 2021;11(7):1716–1735. doi: 10.1158/2159-8290.CD-20-1351. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

