Genomics and pathogenesis of the avian coronavirus infectious bronchitis virus
- PMID: 35978541
- PMCID: PMC9804484
- DOI: 10.1111/avj.13197
Genomics and pathogenesis of the avian coronavirus infectious bronchitis virus
Abstract
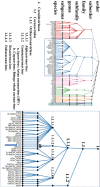
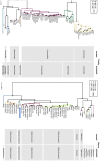
Infectious bronchitis virus (IBV) is a member of the family Coronaviridae, together with viruses such as SARS-CoV, MERS-CoV and SARS-CoV-2 (the causative agent of the COVID-19 global pandemic). In this family of viruses, interspecies transmission has been reported, so understanding their pathobiology could lead to a better understanding of the emergence of new serotypes. IBV possesses a single-stranded, non-segmented RNA genome about 27.6 kb in length that encodes several non-structural and structural proteins. Most functions of these proteins have been confirmed in IBV, but some other proposed functions have been based on research conducted on other members of the family Coronaviridae. IBV has variable tissue tropism depending on the strain, and can affect the respiratory, reproductive, or urinary tracts; however, IBV can also replicate in other organs. Additionally, the pathogenicity of IBV is also variable, with some strains causing only mild clinical signs, while infection with others results in high mortality rates in chickens. This paper extensively and comprehensibly reviews general aspects of coronaviruses and, more specifically, IBV, with emphasis on protein functions and pathogenesis. The pathogenicity of the Australian strains of IBV is also reviewed, describing the variability between the different groups of strains, from the classical to the novel and recombinant strains. Reverse genetic systems, cloning and cell culture growth techniques applicable to IBV are also reviewed.
Keywords: IBV; avian coronavirus; cloning; genetics; infectious bronchitis; pathogenesis; protein functions.
© 2022 The Authors. Australian Veterinary Journal published by John Wiley & Sons Australia, Ltd on behalf of Australian Veterinary Association.
Conflict of interest statement
Glenn F. Browning is an Editorial Board member of the journal and co‐author of this article. He was excluded from the peer‐review process and all editorial decisions related to the acceptance and publication of this article. Peer review was handled independently by members of the Editorial Board to minimise bias.
Figures

Similar articles
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
-
Infectious bronchitis virus in Australia: a model of coronavirus evolution - a review.Avian Pathol. 2021 Aug;50(4):295-310. doi: 10.1080/03079457.2021.1939858. Epub 2021 Jul 15. Avian Pathol. 2021. PMID: 34126817 Review.
-
Construction of an infectious bronchitis virus vaccine strain carrying chimeric S1 gene of a virulent isolate and its pathogenicity analysis.Appl Microbiol Biotechnol. 2020 Oct;104(19):8427-8437. doi: 10.1007/s00253-020-10834-2. Epub 2020 Aug 19. Appl Microbiol Biotechnol. 2020. PMID: 32813067 Free PMC article.
-
Recombinant live attenuated avian coronavirus vaccines with deletions in the accessory genes 3ab and/or 5ab protect against infectious bronchitis in chickens.Vaccine. 2018 Feb 14;36(8):1085-1092. doi: 10.1016/j.vaccine.2018.01.017. Vaccine. 2018. PMID: 29366709 Free PMC article.
-
Deletion of the s2m RNA Structure in the Avian Coronavirus Infectious Bronchitis Virus and Human Astrovirus Results in Sequence Insertions.J Virol. 2023 Mar 30;97(3):e0003823. doi: 10.1128/jvi.00038-23. Epub 2023 Feb 13. J Virol. 2023. PMID: 36779761 Free PMC article.
Cited by
-
Significant Increase of Cinnamic Acid in Metabolites of Chicks Infected with Infectious Bronchitis Virus and Its Remarkable Antiviral Effects In Vitro and In Vivo.Microorganisms. 2025 Jul 10;13(7):1633. doi: 10.3390/microorganisms13071633. Microorganisms. 2025. PMID: 40732142 Free PMC article.
-
Salmonella typhimurium Vaccine Candidate Delivering Infectious Bronchitis Virus S1 Protein to Induce Protection.Biomolecules. 2024 Jan 20;14(1):133. doi: 10.3390/biom14010133. Biomolecules. 2024. PMID: 38275762 Free PMC article.
-
Aerosol Dynamics in the Respiratory Tract of Food-Producing Animals: An Insight into Transmission Patterns and Deposition Distribution.Animals (Basel). 2025 May 12;15(10):1396. doi: 10.3390/ani15101396. Animals (Basel). 2025. PMID: 40427273 Free PMC article.
-
Five Species of Wild Freshwater Sport Fish in Wisconsin, USA, Reveal Highly Diverse Viromes.Pathogens. 2024 Feb 7;13(2):150. doi: 10.3390/pathogens13020150. Pathogens. 2024. PMID: 38392888 Free PMC article.
-
Potential Transcriptional Enhancers in Coronaviruses: From Infectious Bronchitis Virus to SARS-CoV-2.Int J Mol Sci. 2024 Jul 23;25(15):8012. doi: 10.3390/ijms25158012. Int J Mol Sci. 2024. PMID: 39125583 Free PMC article.
References
-
- Beral V, Peterman TA, Berkelman RL et al. Kaposi's sarcoma among persons with AIDS: A sexually transmitted infection? Lancet 1990;335:123–128. - PubMed
-
- Hymes K, Greene J, Marcus A et al. KAPOSI'S sarcoma in homosexual men—A report of eight cases. Lancet 1981;318:598–600. - PubMed
-
- Chua KB, Bellini WJ, Rota PA et al. Nipah virus: A recently emergent deadly paramyxovirus. Science 2000;288:1432–1435. - PubMed
-
- Field H, Young P, Yob JM et al. The natural history of Hendra and Nipah viruses. Microbes Infect 2001;3:307–314. - PubMed
-
- Murray K, Selleck P, Hooper P et al. A morbillivirus that caused fatal disease in horses and humans. Science 1995;268:94–97. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

