A Meta-Analysis on Clinical Outcomes of Ceftolozane versus Piperacillin in Combination with Tazobactam in Patients with Complicated Urinary Tract Infections
- PMID: 35978637
- PMCID: PMC9377909
- DOI: 10.1155/2022/1639114
A Meta-Analysis on Clinical Outcomes of Ceftolozane versus Piperacillin in Combination with Tazobactam in Patients with Complicated Urinary Tract Infections
Abstract
Objective: To evaluate efficacy and adverse events of ceftolozane/tazobactam in complicated UTI including acute pyelonephritis.
Method: Databases that include PubMed, Embase, Scopus, and TRIP were searched. All randomized controlled trials and cohort studies were considered for the study. Statistical analysis was done using a fixed effects model, and results were expressed in proportion for dichotomous data and risk ratio for continuous data with 95% confidence intervals (CI).
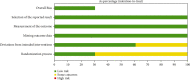
Results: A clinical cure of ceftolozane/tazobactam was found to be 92% with 95% CI of 90-94 while that of piperacillin/tazobactam was only 78% (95% CI, 74-82) in patients with complicated UTI. Microbiological eradication was still higher in the ceftolozane/tazobactam group (83%, 95% CI 81-88) when compared with piperacillin/tazobactam (63% 95% CI, 58.77-65.2). Ceftolozane/tazobactam was more effective in the treatment of complicated urinary tract infections other than acute pyelonephritis as compared to piperacillin/tazobactam (RR = 1.21, 95% CI, 1.07-1.23). Serious adverse events were found comparable in both groups (RR = 1.15, 95% CI, 0.64-2.09).
Conclusion: The analysis showed that ceftolozane/tazobactam has better clinical outcomes including cure rates and low resistance for the treatment of complicated urinary tract infection.
Copyright © 2022 Muhammad Waqas Saeed et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Effect of Cefepime/Enmetazobactam vs Piperacillin/Tazobactam on Clinical Cure and Microbiological Eradication in Patients With Complicated Urinary Tract Infection or Acute Pyelonephritis: A Randomized Clinical Trial.JAMA. 2022 Oct 4;328(13):1304-1314. doi: 10.1001/jama.2022.17034. JAMA. 2022. PMID: 36194218 Free PMC article. Clinical Trial.
-
Analysis of patients with diabetes and complicated intra-abdominal infection or complicated urinary tract infection in phase 3 trials of ceftolozane/tazobactam.BMC Infect Dis. 2017 May 2;17(1):316. doi: 10.1186/s12879-017-2414-9. BMC Infect Dis. 2017. PMID: 28464828 Free PMC article. Clinical Trial.
-
Comparing ceftolozane/tazobactam versus piperacillin/tazobactam as empiric therapy for complicated urinary tract infection in Taiwan: A cost-utility model focusing on gram-negative bacteria.J Microbiol Immunol Infect. 2019 Oct;52(5):807-815. doi: 10.1016/j.jmii.2019.04.003. Epub 2019 Apr 16. J Microbiol Immunol Infect. 2019. PMID: 31029529
-
The use of ceftolozane-tazobactam in the treatment of complicated intra-abdominal infections and complicated urinary tract infections-A meta-analysis of randomized controlled trials.Int J Antimicrob Agents. 2020 Feb;55(2):105858. doi: 10.1016/j.ijantimicag.2019.11.015. Epub 2019 Nov 28. Int J Antimicrob Agents. 2020. PMID: 31786332
-
Ceftolozane/Tazobactam: A Review in Complicated Intra-Abdominal and Urinary Tract Infections.Drugs. 2016 Feb;76(2):231-42. doi: 10.1007/s40265-015-0524-5. Drugs. 2016. PMID: 26746849 Review.
References
-
- Shigemura K., Arakawa S., Tanaka K., Fujisawa M. Clinical investigation of isolated bacteria from urinary tracts of hospitalized patients and their susceptibilities to antibiotics. Journal of Infection and Chemotherapy . 2020;15(1):18–22. https://pubmed.ncbi.nlm.nih.gov/19280295/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

