Novel CA(1-7)M(2-9) Analogs: Synthesis, Characterization, and Antibacterial Evaluation
- PMID: 35978681
- PMCID: PMC9377004
- DOI: 10.1021/acsmedchemlett.2c00270
Novel CA(1-7)M(2-9) Analogs: Synthesis, Characterization, and Antibacterial Evaluation
Abstract
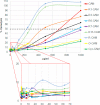
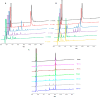
Hybrid peptides from cecropin A and melittin have attracted the interest of the research community for decades. Here we synthesized several new analogs of the pentadecapeptide CA(1-7)M(2-9) and studied their antibacterial and hemolytic activity and tryptic stability. Single substitution of the Lys residues by Arg did not have a significant impact on the antibacterial activity of these analogs, but the substitution of the five Lys residues by Arg resulted in an increment in hemolytic activity. In contrast, the substitution of Lys residues by Orn conserved the antibacterial activity, with even lower hemolysis, and improved the enzymatic stability. The disulfide cyclic version of CA(1-7)M(2-9) was obtained by adding a Cys residue to each end of the peptide and carrying out a chemoselective thiol-disulfide interchange using sec-isoamylmecaptan as protecting group of one of these residues. This cyclic peptide showed good antibacterial activity with low hemolysis and improved enzymatic stability.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources

