Case report: 125I seed implantation for rare malignant solitary fibrous tumor in the pelvic cavity: a case report
- PMID: 35978802
- PMCID: PMC9376293
- DOI: 10.3389/fonc.2022.884491
Case report: 125I seed implantation for rare malignant solitary fibrous tumor in the pelvic cavity: a case report
Abstract
Solitary fibrous tumor (SFT) is a rare spindle cell tumor, benign or low-grade malignant, with an extremely low possibility of occurrence of malignant solitary fibrous tumor (MSFT). Surgery is an effective way for treating SFT, but it is often difficult to resect completely due to a large size, with a high recurrence rate and mortality rate after operation. Additionally, SFT is relatively resistant to chemotherapy, and there is a lack of effective systemic drug treatment. These lead to certain difficulties in the treatment of SFT. We report a case of a rare MSFT in the pelvic cavity. With a history of recurrence after two surgeries, this patient underwent surgical removal combined with 125I seed implantation at our hospital in the context that the tumor could not be completely removed because it was large and adhered to surrounding tissues; after up to 43 months of progression-free survival (PFS), the patient underwent 125I seed implantation alone, and achieved a complete remission, with a PFS up to 35 months. 125I seed implantation can be a safe and effective treatment option for unresectable MSFT as well as a potential solution to repeated local recurrence.
Keywords: 125I seeds; MSFT; brachytherapy; malignant solitary fibrous tumor; recurrence.
Copyright © 2022 Gao, Yu, Di, Zhao, Liang, Liu, Wang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


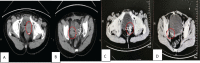
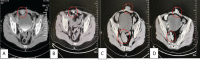
References
-
- Martin-Broto J, Stacchiotti S, Lopez-Pousa A, Redondo A, Bernabeu D, de Alava E, et al. Pazopanib for treatment of advanced malignant and dedifferentiated solitary fibrous tumour: a multicentre, single-arm, phase 2 trial. Lancet Oncol (2019) 20(1):134–44. doi: 10.1016/S1470-2045(18)30676-4 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

