circ-Katnal1 Enhances Inflammatory Pyroptosis in Sepsis-Induced Liver Injury through the miR-31-5p/GSDMD Axis
- PMID: 35979014
- PMCID: PMC9377930
- DOI: 10.1155/2022/8950130
circ-Katnal1 Enhances Inflammatory Pyroptosis in Sepsis-Induced Liver Injury through the miR-31-5p/GSDMD Axis
Abstract
Background: Sepsis is a systemic inflammatory response that can elicit organ dysfunction as well as circulatory diseases in serious cases. When inflammatory responses are especially dysregulated, severe complications can arise, including sepsis-induced liver injury. Various microRNAs along with circular (circ) RNAs are involved in inflammatory responses; nevertheless, their functions in regulating sepsis-induced liver injury remain unknown. The cecal ligation and puncture (CLP) procedure can induce liver injury as well as polymicrobial sepsis.
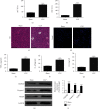
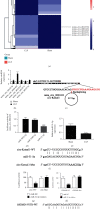
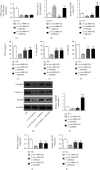
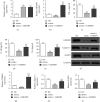
Methods: In this study, CLP was used to induce liver injury as well as polymicrobial sepsis. Then, liver function, inflammatory cytokine expression, and hepatic histopathology were evaluated. High-throughput sequencing was employed to investigate the abnormal hepatic circRNA expression after CLP. Raw264.7 cells were utilized to simulation an in vitro sepsis inflammation model with LPS induce. The relative mRNA as well as protein levels of TNF-α, IL-1β, and IL-6 was explored by quantitative polymerase chain reaction (PCR) and enzyme-linked immunosorbent assays. We explored functional connections among circRNAs, miR-31-5p, and gasdermin D (GSDMD) using dual-luciferase reporter assays. Western blot was employed to test GSDMD, caspase-1, and NLRP3 expression in mice and cell models.
Results: Our results showed that CLP-induced sepsis promoted liver injury via increasing inflammatory pyroptosis. The abnormal expression of circ-Katnal1 played an important role in CLP-induced sepsis. Downregulating circ-Katnal1 suppressed LPS-induced inflammatory pyroptosis in Raw264.7 cells. Bioinformatics and luciferase reporter results confirmed that miR-31-5p and GSDMD were downstream targets of circ-Katnal1. Inhibiting miR-31-5p or upregulating GSDMD reversed the protective effects of silencing circ-Katnal1.
Conclusion: Taken together, circ-Katnal1 enhanced inflammatory pyroptosis in sepsis-induced liver injury through the miR-31-5p/GSDMD axis.
Copyright © 2022 Kai Kang et al.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
Similar articles
-
Liver injury in sepsis: manifestations, mechanisms and emerging therapeutic strategies.Front Immunol. 2025 Mar 28;16:1575554. doi: 10.3389/fimmu.2025.1575554. eCollection 2025. Front Immunol. 2025. PMID: 40226624 Free PMC article. Review.
-
[The role of miR-135b-5p in inhibiting mice acute lung injury (ALI) induced by sepsis and its mechanism].Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022 Jul;38(4):366-372. doi: 10.12047/j.cjap.6263.2022.069. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2022. PMID: 36414563 Chinese.
-
Circ_KATNAL1 promotes the inflammation and apoptosis in human middle ear epithelial cells induced by lipopolysaccharide by regulating the miR-153-3p / TLR4 axis.Cell Mol Biol (Noisy-le-grand). 2023 Aug 31;69(8):172-178. doi: 10.14715/cmb/2023.69.8.26. Cell Mol Biol (Noisy-le-grand). 2023. PMID: 37715400
-
CircRNA_0075723 protects against pneumonia-induced sepsis through inhibiting macrophage pyroptosis by sponging miR-155-5p and regulating SHIP1 expression.Front Immunol. 2023 Feb 27;14:1095457. doi: 10.3389/fimmu.2023.1095457. eCollection 2023. Front Immunol. 2023. PMID: 36923408 Free PMC article.
-
Mechanisms of sepsis-induced acute liver injury: a comprehensive review.Front Cell Infect Microbiol. 2025 Feb 21;15:1504223. doi: 10.3389/fcimb.2025.1504223. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40061452 Free PMC article. Review.
Cited by
-
The Regulatory Role of NcRNAs in Pyroptosis and Disease Pathogenesis.Cell Biochem Biophys. 2025 Apr 18. doi: 10.1007/s12013-025-01720-7. Online ahead of print. Cell Biochem Biophys. 2025. PMID: 40249522 Review.
-
Circ-KATNAL1 Knockdown Reduces Neuronal Apoptosis and Alleviates Spinal Cord Injury Through the miR-98-5p/PRDM5 Regulatory Axis.Mol Biotechnol. 2024 Oct;66(10):2841-2849. doi: 10.1007/s12033-023-00895-9. Epub 2023 Sep 27. Mol Biotechnol. 2024. PMID: 37758970
-
circACTA2 inhibits NLRP3 inflammasome-mediated inflammation via interacting with NF-κB in vascular smooth muscle cells.Cell Mol Life Sci. 2023 Jul 27;80(8):229. doi: 10.1007/s00018-023-04840-6. Cell Mol Life Sci. 2023. PMID: 37498354 Free PMC article.
-
Circular RNA hsa_circ_0000175 Serves as a Potential Biomarker for Rheumatoid Arthritis via miR-31-5p/GSDME Axis.Biochem Genet. 2024 Aug;62(4):2522-2539. doi: 10.1007/s10528-023-10576-6. Epub 2023 Nov 15. Biochem Genet. 2024. PMID: 37968534
-
Liver injury in sepsis: manifestations, mechanisms and emerging therapeutic strategies.Front Immunol. 2025 Mar 28;16:1575554. doi: 10.3389/fimmu.2025.1575554. eCollection 2025. Front Immunol. 2025. PMID: 40226624 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

