Update on the limited sensitivity of computed tomography relative to RT-PCR for COVID-19: a systematic review
- PMID: 35979154
- PMCID: PMC9373863
- DOI: 10.5114/pjr.2022.118238
Update on the limited sensitivity of computed tomography relative to RT-PCR for COVID-19: a systematic review
Abstract
Purpose: The global and ongoing COVID-19 outbreak has compelled the need for timely and reliable methods of detection for SARS-CoV-2 infection. Although reverse transcription-polymerase chain reaction (RT-PCR) has been widely accepted as a reference standard for COVID-19 diagnosis, several early studies have suggested the superior sensitivity of computed tomography (CT) in identifying SARS-CoV-2 infection. In a previous systematic review, we stratified studies based on risk for bias to evaluate the true sensitivity of CT for detecting SARS-CoV-2 infection. This study revisits our prior analysis, incorporating more current data to assess the sensitivity of CT for COVID-19.
Material and methods: The PubMed and Google Scholar databases were searched for relevant articles published between 1 January 2020, and 25 April 2021. Exclusion criteria included lack of specification regarding whether the study cohort was adult or paediatric, whether patients were symptomatic or asymptomatic, and not identifying the source of RT-PCR specimens. Ultimately, 62 studies were included for systematic review and were subsequently stratified by risk for bias using the QUADAS-2 quality assessment tool. Sensitivity data were extracted for random effects meta-analyses.
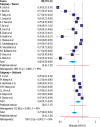
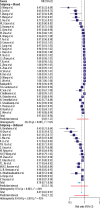
Results: The average sensitivity for COVID-19 reported by the high-risk-of-bias studies was 68% [CI: 58, 80; range: 38-96%] for RT-PCR and 91% [CI: 87, 96; range: 47-100%] for CT. The average sensitivity reported by the low-risk-of-bias studies was 84% [CI: 0.75, 0.94; range: 70-97%] for RT-PCR and 78% [CI: 71, 0.86; range: 44-92%] for CT.
Conclusions: On average, the high-risk-of bias studies underestimated the sensitivity of RT-PCR and overestimated the sensitivity of CT for COVID-19. Given the incorporation of recently published low-risk-of-bias articles, the sensitivities according to low-risk-of-bias studies for both RT-PCR and CT were higher than previously reported.
Keywords: COVID-19; CT; RT-PCR; SARS-CoV-2; sensitivity; systematic review.
Copyright © Polish Medical Society of Radiology 2022.
Figures
References
-
- Waller JV, Allen IE, Lin KK, et al. The limited sensitivity of chest computed tomography relative to reverse transcription polymerase chain reaction for severe acute respiratory syndrome coronavirus-2 infection: a systematic review on COVID-19 diagnostics. Invest Radiol 2020; 55: 754-761. - PMC - PubMed
-
- Runge VM, Heverhagen JT. Scientific advances, investigative radiology 2020 (and beyond). Invest Radiol 2021; 56: 271-273. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
