Two-Photon Polymerization of 2.5D and 3D Microstructures Fostering a Ramified Resting Phenotype in Primary Microglia
- PMID: 35979173
- PMCID: PMC9376863
- DOI: 10.3389/fbioe.2022.926642
Two-Photon Polymerization of 2.5D and 3D Microstructures Fostering a Ramified Resting Phenotype in Primary Microglia
Abstract
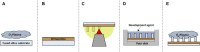
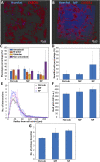
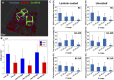
Microglia are the resident macrophages of the central nervous system and contribute to maintaining brain's homeostasis. Current 2D "petri-dish" in vitro cell culturing platforms employed for microglia, are unrepresentative of the softness or topography of native brain tissue. This often contributes to changes in microglial morphology, exhibiting an amoeboid phenotype that considerably differs from the homeostatic ramified phenotype in healthy brain tissue. To overcome this problem, multi-scale engineered polymeric microenvironments are developed and tested for the first time with primary microglia derived from adult rhesus macaques. In particular, biomimetic 2.5D micro- and nano-pillar arrays (diameters = 0.29-1.06 µm), featuring low effective shear moduli (0.25-14.63 MPa), and 3D micro-cages (volume = 24 × 24 × 24 to 49 × 49 × 49 μm3) with and without micro- and nano-pillar decorations (pillar diameters = 0.24-1 µm) were fabricated using two-photon polymerization (2PP). Compared to microglia cultured on flat substrates, cells growing on the pillar arrays exhibit an increased expression of the ramified phenotype and a higher number of primary branches per ramified cell. The interaction between the cells and the micro-pillar-decorated cages enables a more homogenous 3D cell colonization compared to the undecorated ones. The results pave the way for the development of improved primary microglia in vitro models to study these cells in both healthy and diseased conditions.
Keywords: 3D scaffold; effective shear modulus; micro-pillars; nano-pillars; primary microglia; two-photon polymerization.
Copyright © 2022 Sharaf, Roos, Timmerman, Kremers, Bajramovic and Accardo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices.Glia. 2001 Mar 1;33(3):256-66. Glia. 2001. PMID: 11241743
-
Mechanical confinement matters: Unveiling the effect of two-photon polymerized 2.5D and 3D microarchitectures on neuronal YAP expression and neurite outgrowth.Mater Today Bio. 2024 Nov 2;29:101325. doi: 10.1016/j.mtbio.2024.101325. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39569166 Free PMC article.
-
The origin and nature of ramified and amoeboid microglia: a historical review and current concepts.Glia. 1993 Jan;7(1):9-18. doi: 10.1002/glia.440070105. Glia. 1993. PMID: 8423067 Review.
-
Regulation of postnatal forebrain amoeboid microglial cell proliferation and development by the transcription factor Runx1.J Neurosci. 2012 Aug 15;32(33):11285-98. doi: 10.1523/JNEUROSCI.6182-11.2012. J Neurosci. 2012. PMID: 22895712 Free PMC article.
-
Morphology of Microglia Across Contexts of Health and Disease.Methods Mol Biol. 2019;2034:13-26. doi: 10.1007/978-1-4939-9658-2_2. Methods Mol Biol. 2019. PMID: 31392674 Review.
Cited by
-
In vitro microglia models: the era of engineered cell microenvironments.Neural Regen Res. 2023 Aug;18(8):1709-1710. doi: 10.4103/1673-5374.363828. Neural Regen Res. 2023. PMID: 36751786 Free PMC article. No abstract available.
-
Magnetically-actuated microcages for cells entrapment, fabricated by laser direct writing via two photon polymerization.Front Bioeng Biotechnol. 2023 Dec 19;11:1273277. doi: 10.3389/fbioe.2023.1273277. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38170069 Free PMC article.
-
Engineered cell culture microenvironments for mechanobiology studies of brain neural cells.Front Bioeng Biotechnol. 2022 Dec 14;10:1096054. doi: 10.3389/fbioe.2022.1096054. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36588937 Free PMC article. Review.
-
High-Precision 3D Printing of Microporous Cochlear Implants for Personalized Local Drug Delivery.J Funct Biomater. 2023 Oct 3;14(10):494. doi: 10.3390/jfb14100494. J Funct Biomater. 2023. PMID: 37888159 Free PMC article.
-
Micro 3D Printing Elastomeric IP-PDMS Using Two-Photon Polymerisation: A Comparative Analysis of Mechanical and Feature Resolution Properties.Polymers (Basel). 2023 Apr 7;15(8):1816. doi: 10.3390/polym15081816. Polymers (Basel). 2023. PMID: 37111964 Free PMC article.
References
-
- Accardo A., Blatché M.-C., Courson R., Loubinoux I., Thibault C., Malaquin L., et al. (2017). Multiphoton Direct Laser Writing and 3D Imaging of Polymeric Freestanding Architectures for Cell Colonization. Small 13, 1700621. 10.1002/smll.201700621 10.1002/smll.201700621 | Google Scholar - DOI - DOI - PubMed
-
- Accardo A., Blatché M.-C., Courson R., Loubinoux I., Vieu C., Malaquin L. (2018). Direct Laser Fabrication of Free-Standing PEGDA-Hydrogel Scaffolds for Neuronal Cell Growth. Mater. Today 21, 315–316. 10.1016/j.mattod.2018.02.004 10.1016/j.mattod.2018.02.004 | Google Scholar - DOI - DOI
-
- Akolawala Q., Rovituso M., Versteeg H. H., Rondon A. M. R., Accardo A. (2022). Evaluation of Proton-Induced DNA Damage in 3D-Engineered Glioblastoma Microenvironments. ACS Appl. Mat. Interfaces 14, 20778–20789. 10.1021/acsami.2c03706 10.1021/acsami.2c03706 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Angeloni L., Ganjian M., Nouri-Goushki M., Mirzaali M. J., Hagen C. W., Zadpoor A. A., et al. (2021). Mechanical Characterization of Nanopillars by Atomic Force Microscopy. Addit. Manuf. 39, 101858. 10.1016/j.addma.2021.101858 10.1016/j.addma.2021.101858 | Google Scholar - DOI - DOI
-
- Antonova O. Y., Kochetkova O. Y., Shlyapnikov Y. M. (2021). ECM-mimetic Nylon Nanofiber Scaffolds for Neurite Growth Guidance. Nanomaterials 11, 516. 10.3390/nano11020516 PubMed Abstract | 10.3390/nano11020516 | Google Scholar - DOI - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

