Differential dynamics of bone graft transplantation and mesenchymal stem cell therapy during bone defect healing in a murine critical size defect
- PMID: 35979174
- PMCID: PMC9357712
- DOI: 10.1016/j.jot.2022.05.010
Differential dynamics of bone graft transplantation and mesenchymal stem cell therapy during bone defect healing in a murine critical size defect
Abstract
Background: A critical size bone defect is a clinical scenario in which bone is lost or excised due to trauma, infection, tumor, or other causes, and cannot completely heal spontaneously. The most common treatment for this condition is autologous bone grafting to the defect site. However, autologous bone graft is often insufficient in quantity or quality for transplantation to these large defects. Recently, tissue engineering methods using mesenchymal stem cells (MSCs) have been proposed as an alternative treatment. However, the underlying biological principles and optimal techniques for tissue regeneration of bone using stem cell therapy have not been completely elucidated.
Methods: In this study, we compare the early cellular dynamics of healing between bone graft transplantation and MSC therapy in a murine chronic femoral critical-size bone defect. We employ high-dimensional mass cytometry to provide a comprehensive view of the differences in cell composition, stem cell functionality, and immunomodulatory activity between these two treatment methods one week after transplantation.
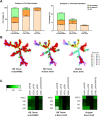
Results: We reveal distinct cell compositions among tissues from bone defect sites compared with original bone graft, show active recruitment of MSCs to the bone defect sites, and demonstrate the phenotypic diversity of macrophages and T cells in each group that may affect the clinical outcome.
Conclusion: Our results provide critical data and future directions on the use of MSCs for treating critical size defects to regenerate bone.Translational Potential of this article: This study showed systematic comparisons of the cellular and immunomodulatory profiles among different interventions to improve the healing of the critical-size bone defect. The results provided potential strategies for designing robust therapeutic interventions for the unmet clinical need of treating critical-size bone defects.
Keywords: Bone graft; Critical-size bone defect; CyTOF; Macrophages; Stem cells; T cells.
© 2022 Published by Elsevier B.V. on behalf of Chinese Speaking Orthopaedic Society.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Figures




References
-
- Hak D.J., Fitzpatrick D., Bishop J.A., Marsh J.L., Tilp S., Schnettler R., et al. Delayed union and nonunions: epidemiology, clinical issues, and financial aspects. Injury. 2014;45(Suppl 2):S3–S7. - PubMed
-
- Burchardt H. Biology of bone transplantation. Orthop Clin N Am. 1987;18(2):187–196. - PubMed
-
- Bunpetch V., Zhang Z.Y., Zhang X., Han S., Zongyou P., Wu H., et al. Strategies for MSC expansion and MSC-based microtissue for bone regeneration. Biomaterials. 2019;196:67–79. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

