Jinhua Qinggan granules for non-hospitalized COVID-19 patients: A double-blind, placebo-controlled, and randomized controlled trial
- PMID: 35979216
- PMCID: PMC9376460
- DOI: 10.3389/fmed.2022.928468
Jinhua Qinggan granules for non-hospitalized COVID-19 patients: A double-blind, placebo-controlled, and randomized controlled trial
Abstract
Background: Key findings from the World Health Organization Expert Meeting on Evaluation of Traditional Chinese Medicine (TCM) in treating coronavirus disease 2019 (COVID-19) reported that TCMs are beneficial, particularly for mild-to-moderate cases. The efficacy of Jinhua Qinggan granules (JHQG) in COVID-19 patients with mild symptoms has yet to be clearly defined.
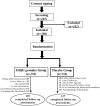
Methods: We conducted a phase 2/3, double-blind, randomized, placebo-controlled trial to evaluate the efficacy and safety of treatment with JHQG in mild, non-hospitalized, laboratory-confirmed COVID-19 patients. Participants were randomly assigned to receive 5 g/sacket of JHQG or placebo granules orally thrice daily for 10 days. The primary outcomes were the improvement in clinical symptoms and a proportion tested negative on viral polymerase chain reaction (PCR) after treatment. Secondary outcomes were the time to recover from clinical symptoms and changes in white blood cells (WBC) and acute phase reactants (C-reactive protein (CRP) and ferritin) on the 10th day after treatment initiation.
Results: A total of 300 patients were randomly assigned to receive JHQG (150 patients) and placebo (150 patients). Baseline characteristics were similar in the two groups. In the modified intention-to-treat analysis, JHQG showed greater clinical efficacy (82.67%) on the 10th day of the trial compared with the placebo group (10.74%; rate difference: 71.93%; 95% CI 64.09-79.76). The proportion of patients with a negative PCR after treatment was comparable (rate difference: -4.67%; 95% CI -15.76 to 6.42). In contrast, all changes in WBC, ferritin, and CRP levels showed a statistically significant decline in JHQG (P ≤ 0.044) after treatment, but not the latter in placebo (P = 0.077). The median time to recovery of COVID-19-related symptoms including cough, sputum, sore throat, dyspnea, headache, nasal obstruction, fatigue, and myalgia was shorter in the JHQG group compared to the placebo group (P < 0.001 for all). Three patients experienced mild-to-moderate adverse events (AEs) duringthe treatment period in the JHQG group. Findings were similar between the modified intention-to-treat and the per-protocol analysis that included only patients who reported 100% adherence to the assigned regimen.
Conclusion: Based on the time to recover from the COVID-19-related symptoms and AEs, it is concluded that JHQG is a safe and effective TCM for symptomatic relief of patients with mild COVID-19. A symptomatic improvement in the JHQG group patients was observed and JHQG use would have important public health implications in such patients.
Clinical trial registration: The Trial was prospectively registered on www.clinicaltrials.gov with registration number: NCT04723524.
Keywords: COVID-19; Chinese mineral medicine; Jinhua Qinggan granules (JHQG); Traditional Chinese Medicine; randomized controlled (clinical) trial.
Copyright © 2022 Shah, Fatima, Khan, Ullah, Himani, Wan, Lin, Lau, Liu and Lam.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Jin-Zhen oral liquid for pediatric coronavirus disease (COVID-19): A randomly controlled, open-label, and non-inferiority trial at multiple clinical centers.Front Pharmacol. 2023 Feb 27;14:1094089. doi: 10.3389/fphar.2023.1094089. eCollection 2023. Front Pharmacol. 2023. PMID: 36923353 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Efficacy and safety of Jinhua Qinggan granules in the treatment of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis.Medicine (Baltimore). 2023 Apr 14;102(15):e33545. doi: 10.1097/MD.0000000000033545. Medicine (Baltimore). 2023. PMID: 37058020 Free PMC article.
-
Therapeutic effects and safety of oral Chinese patent medicine for COVID-19: A rapid systematic review and meta-analysis of randomized controlled trials.Complement Ther Med. 2021 Aug;60:102744. doi: 10.1016/j.ctim.2021.102744. Epub 2021 Jun 6. Complement Ther Med. 2021. PMID: 34091029 Free PMC article.
Cited by
-
Network-based pharmacology and UHPLC-Q-Exactive-Orbitrap-MS reveal Jinhua Qinggan granule's mechanism in reducing cellular inflammation in COVID-19.Front Immunol. 2024 Jul 4;15:1382524. doi: 10.3389/fimmu.2024.1382524. eCollection 2024. Front Immunol. 2024. PMID: 39026676 Free PMC article.
-
Practice and principle of traditional Chinese medicine for the prevention and treatment of COVID-19.Front Med. 2023 Dec;17(6):1014-1029. doi: 10.1007/s11684-023-1040-8. Epub 2023 Dec 29. Front Med. 2023. PMID: 38157191 Review.
-
Initial observations of Jinhua Qinggan Granules, a Chinese medicine, in the mitigation of hospitalization and mortality in high-risk elderly with COVID-19 infection: A retrospective study in an old age home in Hong Kong.Front Med (Lausanne). 2022 Jul 27;9:948149. doi: 10.3389/fmed.2022.948149. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35966846 Free PMC article. No abstract available.
-
Effect of Chinese Medicine in Patients with COVID-19: A Multi-center Retrospective Cohort Study.Chin J Integr Med. 2024 Nov;30(11):974-983. doi: 10.1007/s11655-024-4108-7. Epub 2024 May 31. Chin J Integr Med. 2024. PMID: 38816638
-
Recent advances towards natural plants as potential inhibitors of SARS-Cov-2 targets.Pharm Biol. 2023 Dec;61(1):1186-1210. doi: 10.1080/13880209.2023.2241518. Pharm Biol. 2023. PMID: 37605622 Free PMC article. Review.
References
-
- Organization WH . COVID-19 Weekly Epidemiological Update, Edition 76, 25 January. (2022). Available online at: https://apps.who.int/iris/handle/10665/351190
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous