Population Pharmacokinetics of Cyclosporine in Chinese Pediatric Patients With Acquired Aplastic Anemia
- PMID: 35979231
- PMCID: PMC9377374
- DOI: 10.3389/fphar.2022.933739
Population Pharmacokinetics of Cyclosporine in Chinese Pediatric Patients With Acquired Aplastic Anemia
Abstract
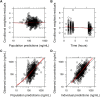
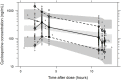
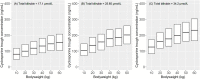
Cyclosporine (CsA) is a component of the first-line treatment for acquired aplastic anemia (acquired AA) in pediatric patients. This study aimed to develop a population pharmacokinetic (PK) model of CsA in Chinese pediatric patients with acquired AA to inform individual dosage regimens. A total of 681 CsA whole blood concentrations and laboratory data of 157 pediatric patients with acquired AA were retrospectively collected from two hospitals in Shanghai. A nonlinear mixed-effect model approach was used to build the population PK model. Potential covariate effects of age, body weight, and biochemical measurements (renal and liver functions) on CsA PK disposition were evaluated. Model fit was assessed using the basic goodness of fit and a visual predictive check. The CsA concentration data were accurately described using a two-compartment disposition model with first-order absorption and elimination. Body weight value was implemented as a fixed allometric function on all clearance and volume of distribution parameters. Total bilirubin level was identified as a significant covariate on apparent clearance (CL/F), with a 1.07% reduction per 1 nmol/L rise in total bilirubin level. The final estimates for CL/F and central volume (Vc/F) were 29.1 L/h and 325 L, respectively, for a typical 28 kg child. Other covariates (e.g., gender, age, albumin, hemoglobin, hematocrit, serum creatinine, and concomitant medication) did not significantly affect the PK properties of CsA. This population PK model, along with a maximum a posteriori Bayesian approach, could estimate individual PK parameters in pediatric patients with acquired AA to conduct individual CsA therapy.
Keywords: NONMEM; acquired aplastic anemia; cyclosporine; pediatric patients; population pharmacokinetics.
Copyright © 2022 Gao, Bian, Qiao, Qian, Li, Shen, Miao, Yu, Meng, Zhu, Jiang, Le, Yu, Wang and Zhai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures
References
-
- Albitar O., Ballouze R., Harun S. N., Mohamed Noor D. A., Sheikh Ghadzi S. M. (2020). Population Pharmacokinetic Modeling of Cyclosporine Among Malaysian Renal Transplant Patients: An Evaluation of Methods to Handle Missing Doses in Conventional Drug-Monitoring Data. J. Clin. Pharmacol. 60, 1474–1482. 10.1002/jcph.1670 PubMed Abstract | 10.1002/jcph.1670 | Google Scholar - DOI - PubMed
-
- Aoyama T., Yamano S., Waxman D. J., Lapenson D. P., Meyer U. A., Fischer V., et al. (1989). Cytochrome P-450 hPCN3, a Novel Cytochrome P-450 IIIA Gene Product that Is Differentially Expressed in Adult Human Liver. cDNA and Deduced Amino Acid Sequence and Distinct Specificities of cDNA-Expressed hPCN1 and hPCN3 for the Metabolism of Steroid Hormones and Cyclosporine. J. Biol. Chem. 264, 10388–10395. 10.1016/s0021-9258(18)81632-5 PubMed Abstract | 10.1016/s0021-9258(18)81632-5 | Google Scholar - DOI - PubMed
-
- Bacigalupo A., Hows J., Gluckman E., Nissen C., Marsh J., Van Lint M. T., et al. (1988). Bone Marrow Transplantation (BMT) versus Immunosuppression for the Treatment of Severe Aplastic Anaemia (SAA): a Report of the EBMT SAA Working Party. Br. J. Haematol. 70, 177–182. 10.1111/j.1365-2141.1988.tb02460.x PubMed Abstract | 10.1111/j.1365-2141.1988.tb02460.x | Google Scholar - DOI - PubMed
-
- Benfield M. R., Stablein D., Tejani A. (1999). Trends in Immunosuppressive Therapy: a Report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr. Transpl. 3, 27–32. 10.1034/j.1399-3046.1999.00001.x 10.1034/j.1399-3046.1999.00001.x | Google Scholar - DOI - PubMed
-
- Bergstrand M., Hooker A. C., Wallin J. E., Karlsson M. O. (2011). Prediction-corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. Aaps J. 13, 143–151. 10.1208/s12248-011-9255-z PubMed Abstract | 10.1208/s12248-011-9255-z | Google Scholar - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

