Vaccine adjuvants to engage the cross-presentation pathway
- PMID: 35979365
- PMCID: PMC9376467
- DOI: 10.3389/fimmu.2022.940047
Vaccine adjuvants to engage the cross-presentation pathway
Abstract
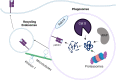
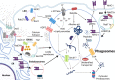
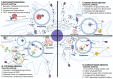
Adjuvants are indispensable components of vaccines for stimulating optimal immune responses to non-replicating, inactivated and subunit antigens. Eliciting balanced humoral and T cell-mediated immunity is paramount to defend against diseases caused by complex intracellular pathogens, such as tuberculosis, malaria, and AIDS. However, currently used vaccines elicit strong antibody responses, but poorly stimulate CD8 cytotoxic T lymphocyte (CTL) responses. To elicit potent CTL memory, vaccines need to engage the cross-presentation pathway, and this requirement has been a crucial bottleneck in the development of subunit vaccines that engender effective T cell immunity. In this review, we focus on recent insights into DC cross-presentation and the extent to which clinically relevant vaccine adjuvants, such as aluminum-based nanoparticles, water-in oil emulsion (MF59) adjuvants, saponin-based adjuvants, and Toll-like receptor (TLR) ligands modulate DC cross-presentation efficiency. Further, we discuss the feasibility of using carbomer-based adjuvants as next generation of adjuvant platforms to elicit balanced antibody- and T-cell based immunity. Understanding of the molecular mechanism of DC cross-presentation and the mode of action of adjuvants will pave the way for rational design of vaccines for infectious diseases and cancer that require balanced antibody- and T cell-based immunity.
Keywords: CD8 T cells; adjuvants; cross-presentation; immunity; memory; vaccines.
Copyright © 2022 Lee and Suresh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

