Supramolecular organizing centers at the interface of inflammation and neurodegeneration
- PMID: 35979366
- PMCID: PMC9377691
- DOI: 10.3389/fimmu.2022.940969
Supramolecular organizing centers at the interface of inflammation and neurodegeneration
Abstract
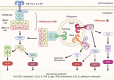
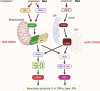
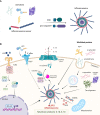
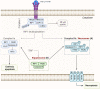
The pathogenesis of neurodegenerative diseases involves the accumulation of misfolded protein aggregates. These deposits are both directly toxic to neurons, invoking loss of cell connectivity and cell death, and recognized by innate sensors that upon activation release neurotoxic cytokines, chemokines, and various reactive species. This neuroinflammation is propagated through signaling cascades where activated sensors/receptors, adaptors, and effectors associate into multiprotein complexes known as supramolecular organizing centers (SMOCs). This review provides a comprehensive overview of the SMOCs, involved in neuroinflammation and neurotoxicity, such as myddosomes, inflammasomes, and necrosomes, their assembly, and evidence for their involvement in common neurodegenerative diseases. We discuss the multifaceted role of neuroinflammation in the progression of neurodegeneration. Recent progress in the understanding of particular SMOC participation in common neurodegenerative diseases such as Alzheimer's disease offers novel therapeutic strategies for currently absent disease-modifying treatments.
Keywords: amyloid deposits; inflammasome; inflammation; myddosome; necrosome; neurodegenerative diseases; neurotoxicity; supramolecular organizing centers.
Copyright © 2022 Sušjan-Leite, Ramuta, Boršić, Orehek and Hafner-Bratkovič.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

